The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1 - PubMed (original) (raw)
The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1
Corinne Cayrol et al. Proc Natl Acad Sci U S A. 2009.
Abstract
IL-33 is a chromatin-associated cytokine of the IL-1 family that has recently been linked to many diseases, including asthma, rheumatoid arthritis, atherosclerosis, and cardiovascular diseases. IL-33 signals through the IL-1 receptor-related protein ST2 and drives production of pro-inflammatory and T helper type 2-associated cytokines in mast cells, T helper type 2 lymphocytes, basophils, eosinophils, invariant natural killer T cells, and natural killer cells. It is currently believed that IL-33, like IL-1beta and IL-18, requires processing by caspase-1 to a mature form (IL-33(112-270)) for biological activity. Contrary to the current belief, we report here that full-length IL-33(1-270) is active and that processing by caspase-1 results in IL-33 inactivation, rather than activation. We show that full-length IL-33(1-270) binds and activates ST2, similarly to IL-33(112-270), and that cleavage by caspase-1 does not occur at the site initially proposed (Ser(111)), but rather after residue Asp(178) between the fourth and fifth predicted beta-strands of the IL-1-like domain. Surprisingly, the caspase-1 cleavage site (DGVD(178)G) is similar to the consensus site of cleavage by caspase-3, and IL-33 is also a substrate for this apoptotic caspase. Interestingly, we found that full-length IL-33, which is constitutively expressed to high levels by endothelial cells in most normal human tissues, can be released in the extracellular space after endothelial cell damage or mechanical injury. We speculate that IL-33 may function, similarly to the prototypical alarmins HMGB1 and IL-1alpha, as an endogenous danger signal to alert cells of the innate immune system of tissue damage during trauma or infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
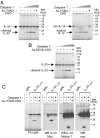
Processing of full-length IL-33 by caspase-1 generates a 20–22 kDa cleavage product that does not correspond to the IL-1-like domain. (A) Recombinant caspase-1 cleaves full-length IL-33 and pro-IL1β in vitro. Fluorescently labeled proteins were incubated for 2 h at 37 °C with increasing amounts of recombinant caspase-1 (0.05, 0.15, 0.5, or 1 unit) and then analyzed by SDS/PAGE and fluorography. Cleavage was abrogated by the caspase-1 inhibitor Ac-YVAD-CHO. (B) Recombinant caspase-3 cleaves full-length IL-33 in vitro. Fluorescently labeled IL-33 was incubated with increasing amounts of recombinant caspase-3 (0.05, 0.15, 0.5, or 1 unit) as described in A. Cleavage was abrogated by the caspase-3 inhibitor Ac-DEVD-CHO. (C) The 20–22 kDa caspase-1 cleavage product of IL-33 is recognized by IL-33 Nter antibodies but not by antibodies to the C terminus (Nessy-1, anti-myc). Fluorescently labeled or unlabeled IL-33 proteins, containing a C-terminal myc-epitope tag, were cleaved with 0.5 unit of caspase-1 as described in A and analyzed by fluorography (fluorescent IL-33) or Western blot (unlabeled IL-33) with IL-33 Nter, Nessy-1, or anti-myc antibodies.
Fig. 2.
Caspase-1 processing of IL-33 occurs after residue Asp178 within the IL-1-like domain. (A) Primary structure of human IL-33. The N-terminal domain involved in IL-33 nuclear activities and the IL-1-like domain, with its 12 predicted β-strands (black boxes), are indicated. The sequence surrounding the caspase-1 and caspase-3 cleavage site (Asp178) is shown for both human (Hs) and mouse (Mm) IL-33. CBM, chromatin-binding motif (aa 40–58). (B) An IL-331–178 deletion protein generated by in vitro translation (not tagged with myc-epitope) co-migrates on SDS/PAGE with the 20–22 kDa caspase-1 cleavage product of IL-33. Fluorescently labeled IL-33 protein was incubated with 0.5 units of caspase-1 for 2 h at 37 °C (with or without prior incubation with Ac-YVAD-CHO inhibitor) and analyzed by SDS/PAGE and fluorography. (C and D) Mutation of Asp178 to alanine abrogates cleavage of IL-33 by both caspase-1 (C) and caspase-3 (D). Fluorescently labeled IL-331–270 and IL-33D178A proteins were incubated with recombinant caspase-1 (C) or caspase-3 (D) as described in B. The Ac-YVAD-CHO and Ac-DEVD-CHO inhibitors were used at 100 μM. Asterisk indicates non-specific band. (E) Mutation of Asp178 to alanine abrogates cleavage of IL-33 by endogenous caspases during doxorubicin-induced apoptosis. U20S cells were transfected with IL-331–270 or IL-33D178A expression vectors and treated 24 h later with doxorubicin in the presence or absence of the pan-caspase inhibitor Z-VAD-fmk. Proteins were analyzed 24 h later by Western blot analysis with IL-33 mAb 305B. (F) Endogenous IL-33 in primary human endothelial cells (treated with control or IL-33 siRNA) was detected by Western blot analysis with IL-33 mAb 305B. (G) Endogenous IL-33 is cleaved by endogenous caspases in endothelial cells treated with the apoptosis-inducing agent staurosporine. Endothelial cells were treated with staurosporine in the presence or absence of the pan-caspase inhibitor Z-VAD-fmk. Proteins were analyzed by Western blot analysis with IL-33 mAb 305B or PARP mAb (used as a control). IL-331–270 and IL-331–178 proteins (not tagged with myc-epitope), generated by in vitro translation, are shown (Right).
Fig. 3.
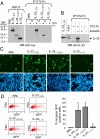
Full-length IL-331–270 is able to bind and activate the ST2 receptor. (A) Pull-down of full-length IL-331–270 with ST2-Fc fusion protein. Full-length (IL-331–270), C-terminal IL-1-like domain (IL-33112–270), and N-terminal domain (IL-331–111) proteins tagged with myc-epitope at their C terminus were incubated with ST2-Fc for 16 h at 4 °C and precipitated with protein-G agarose beads. The precipitates were separated by SDS/PAGE and analyzed by Western blot with anti-myc antibody. Rabbit reticulocyte lysate (RRL) is an un-programmed lysate. (B) Pull-down of endogenous IL-33 with ST2-Fc fusion protein. Endothelial cell freeze-thaw extracts were incubated with ST2-Fc and the precipitates were analyzed by Western blot with IL-33 mAb 305B. Asterisk indicates non-specific band. (C and D) Full-length IL-331–270 activates an ST2-dependent NFκB-GFP reporter gene. Assays were performed in HEK293T cells transfected with plasmids pNF-κB-hrGFP and pEF-BOS-hST2, using in vitro translated IL-331–270, IL-33112–270, and IL-331–111 proteins, as described in Materials and Methods. Cells were analyzed for GFP expression by fluorescence microscopy (C) and flow cytometry (D). The percentage increase in GFP+ cells is shown (Below). Results are shown as means and SDs of 3 independent transfection experiments.
Fig. 4.
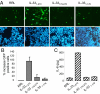
The 2 caspase-1 cleavage products, IL-331–178 and IL-33179–270, are not able to activate ST2. (A and B) The capacity of IL-331–178 and IL-33179–270 to activate ST2-dependent signaling was analyzed using an ST2-dependent NFκB-GFP reporter gene. Assays were performed in HEK293T cells transfected with plasmids pNF-κB-hrGFP and pEF-BOS-hST2 using in vitro translated IL-331–270, IL-33179–270, and IL-331–178 proteins as described in Materials and Methods. Cells were analyzed for GFP expression by fluorescence microscopy (A) and flow cytometry (B). The percentage increase in GFP+ cells is shown (Below). Results are shown as means and SDs of 3 independent transfection experiments. (C) The capacity of IL-33 and deletion mutants to activate the IL-33-responsive mast cell line MC/9 was analyzed by determining IL-6 levels in supernatants using an ELISA. Results are shown as means and SDs of 3 separate data points.
Fig. 5.
Full-length IL-33 is released by damaged endothelial cells. (A-D) Western blot analysis of confluent primary human endothelial cells lysates or supernatants was performed using antibodies against IL-33 (AT-110) and HMGB1. (A) Similar amounts of HMGB1 were observed in the presence or absence of siRNA to IL-33. (B) IL-33 and HMGB1 were released in the supernatants after scraping of the cells from the substratum (followed by 20 min incubation at 37 °C) or scratching the endothelial monolayer with a surgical scalpel. Supernatants were collected from wounded cells and the presence of IL-33 and HMGB1 was assayed in both pellets and supernatants concentrated by TCA precipitation or filtration on Vivaspin columns. Asterisk indicates non-specific band. (C) Higher amounts of IL-33 and HMGB1 were released in the supernatants after endothelial cell damage induced by repeated cycles of freezing and thawing. (D) IL-33 and HMGB1 were also released in the supernatants after treatment of the endothelial cells for 5 min at 37 °C with non-ionic detergents 0.2% Nonidet P-40 and 0.2% Triton X-100.
Similar articles
- IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G.
Lefrançais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. Lefrançais E, et al. Proc Natl Acad Sci U S A. 2012 Jan 31;109(5):1673-8. doi: 10.1073/pnas.1115884109. Epub 2012 Jan 17. Proc Natl Acad Sci U S A. 2012. PMID: 22307629 Free PMC article. - Interleukin-33 is biologically active independently of caspase-1 cleavage.
Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Talabot-Ayer D, et al. J Biol Chem. 2009 Jul 17;284(29):19420-6. doi: 10.1074/jbc.M901744200. Epub 2009 May 22. J Biol Chem. 2009. PMID: 19465481 Free PMC article. - The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'?
Moussion C, Ortega N, Girard JP. Moussion C, et al. PLoS One. 2008 Oct 6;3(10):e3331. doi: 10.1371/journal.pone.0003331. PLoS One. 2008. PMID: 18836528 Free PMC article. - The interleukin (IL)-1 cytokine family--Balance between agonists and antagonists in inflammatory diseases.
Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. Palomo J, et al. Cytokine. 2015 Nov;76(1):25-37. doi: 10.1016/j.cyto.2015.06.017. Epub 2015 Jul 13. Cytokine. 2015. PMID: 26185894 Review. - The enigmatic processing and secretion of interleukin-33.
Zhao W, Hu Z. Zhao W, et al. Cell Mol Immunol. 2010 Jul;7(4):260-2. doi: 10.1038/cmi.2010.3. Epub 2010 Mar 22. Cell Mol Immunol. 2010. PMID: 20305685 Free PMC article. Review.
Cited by
- Opposing Functions of Classic and Novel IL-1 Family Members in Gut Health and Disease.
Lopetuso LR, Chowdhry S, Pizarro TT. Lopetuso LR, et al. Front Immunol. 2013 Jul 9;4:181. doi: 10.3389/fimmu.2013.00181. eCollection 2013. Front Immunol. 2013. PMID: 23847622 Free PMC article. - Role of IL-33-ST2 pathway in regulating inflammation: current evidence and future perspectives.
Zhou Y, Xu Z, Liu Z. Zhou Y, et al. J Transl Med. 2023 Dec 11;21(1):902. doi: 10.1186/s12967-023-04782-4. J Transl Med. 2023. PMID: 38082335 Free PMC article. Review. - Altered Treg and cytokine responses in RSV-infected infants.
Christiaansen AF, Syed MA, Ten Eyck PP, Hartwig SM, Durairaj L, Kamath SS, Varga SM. Christiaansen AF, et al. Pediatr Res. 2016 Nov;80(5):702-709. doi: 10.1038/pr.2016.130. Epub 2016 Jun 21. Pediatr Res. 2016. PMID: 27486703 Free PMC article. - Extracellular IL-33 cytokine, but not endogenous nuclear IL-33, regulates protein expression in endothelial cells.
Gautier V, Cayrol C, Farache D, Roga S, Monsarrat B, Burlet-Schiltz O, Gonzalez de Peredo A, Girard JP. Gautier V, et al. Sci Rep. 2016 Oct 3;6:34255. doi: 10.1038/srep34255. Sci Rep. 2016. PMID: 27694941 Free PMC article. - Interleukin-33 in the human placenta.
Topping V, Romero R, Than NG, Tarca AL, Xu Z, Kim SY, Wang B, Yeo L, Kim CJ, Hassan SS, Kim JS. Topping V, et al. J Matern Fetal Neonatal Med. 2013 Mar;26(4):327-38. doi: 10.3109/14767058.2012.735724. Epub 2012 Nov 23. J Matern Fetal Neonatal Med. 2013. PMID: 23039129 Free PMC article.
References
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. - PubMed
- Dinarello CA. Interleukin-18, a proinflammatory cytokine. Eur Cytokine Netw. 2000;11:483–486. - PubMed
- Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous