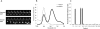MS-qFRET: a quantum dot-based method for analysis of DNA methylation - PubMed (original) (raw)
MS-qFRET: a quantum dot-based method for analysis of DNA methylation
Vasudev J Bailey et al. Genome Res. 2009 Aug.
Abstract
DNA methylation contributes to carcinogenesis by silencing key tumor suppressor genes. Here we report an ultrasensitive and reliable nanotechnology assay, MS-qFRET, for detection and quantification of DNA methylation. Bisulfite-modified DNA is subjected to PCR amplification with primers that would differentiate between methylated and unmethylated DNA. Quantum dots are then used to capture PCR amplicons and determine the methylation status via fluorescence resonance energy transfer (FRET). Key features of MS-qFRET include its low intrinsic background noise, high resolution, and high sensitivity. This approach detects as little as 15 pg of methylated DNA in the presence of a 10,000-fold excess of unmethylated alleles, enables reduced use of PCR (as low as eight cycles), and allows for multiplexed analyses. The high sensitivity of MS-qFRET enables one-step detection of methylation at PYCARD, CDKN2B, and CDKN2A genes in patient sputum samples that contain low concentrations of methylated DNA, which normally would require a nested PCR approach. The direct application of MS-qFRET on clinical samples offers great promise for its translational use in early cancer diagnosis, prognostic assessment of tumor behavior, as well as monitoring response to therapeutic agents.
Figures
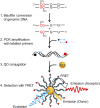
Figure 1.
Principle of MS-qFRET for detection of DNA methylation. In step 1, extracted genomic DNA is subject to sodium bisulfite conversion, wherein unmethylated cytosines are converted to uracil while methylated cytosines remain unaffected. In step 2, DNA is amplified using PCR wherein the forward and reverse primers are labeled with a biotin (black dot) and a fluorophore (red dot), respectively. In step 3, the resulting labeled-PCR product is captured by streptavidin functionalized QDs through streptavidin-biotin affinity. Finally, in step 4, upon suitably exciting the QD, the nanoassembly formed allows for FRET to occur between the QD donor and the fluorophore acceptor. Consequently, the labeled-PCR products are detected by emissions of fluorophores accompanied by quenching of QDs to reveal the status of DNA methylation.
Figure 2.
High analytical sensitivity facilitated by inherent low-background noise. (A) Methylation for CDKN2A can be detected as early as eight cycles as demonstrated by the FRET efficiency, which is significantly higher than that of water control. FRET efficiency from the standard 35-cycle control is much higher due to both a stronger acceptor emission accompanied by stronger QD quenching. Error bars are computed from five separate experiments. (B) Corresponding MSP gel readout indicates no visible band at eight cycles for methylated CDKN2A product but a clear band for the standard 35 cycles. (C) Confocal spectroscopy is used to observe differences in the positive control (IVD only) and negative control (NL only) through 2000-msec single-particle traces. (Top) In positive control, each Cy5 peak seen is the fluorescence burst associated with labeled-MSP products that is linked to a single QD passing through the focal detection volume of a confocal spectroscopy setup. (Bottom) The negative control has very low background noise. (D) IVD was serially diluted in NL DNA (150 ng) and subject to MS-qFRET with 40 cycles of amplification. Confocal spectroscopy is used to analyze fluorescent bursts for the acceptor (Cy5) and was plotted for the entire time duration (three separate runs of 100 sec) for 1/10, 1/100, 1/1000, and 1/10,000 and 0 methylated/unmethylated CDKN2A alleles (IVD/NL). This indicates the successful detection of methylation with as little as 15 pg of methylated DNA (∼5 genomic equivalents) in 150 ng of excess unmethylated DNA.
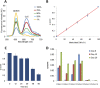
Figure 3.
Quantitative ability of MS-qFRET. (A) Experiments with different ratios of unmethylated and methylated DNA (see Methods) show that increasing percent CDKN2A methylation levels are accompanied by an increase in acceptor (Cy5) emission at 670 nm and corresponding donor (QD605) quenching at 605 nm. (B) _q_-scores are plotted for the varying levels of CDKN2A methylation and a linear fit is observed with _r_2 = 0.999. Results are plotted from five separate mixing experiments. (C) Quantitative ability of MS-qFRET to estimate CDKN2A methylation reversal in DNA from RKO cells treated with DAC for different time points. Error bars are generated from three separate repetitions. _q_-scores indicate a drop in the level of methylation post-treatment. (D) Quantification of methylation reversal at CDKN2B using MS-qFRET in six MDS patients using 150 ng of input DNA during their first cycle of epigenetic therapy. Changes in levels of methylation are effectively captured to show varying cellular responses to therapy with 5-azacytidine and MS-275.
Figure 4.
Detection of methylation in human sputum samples. (A) Representative gel from sputum DNA using conventional MSP for PYCARD for eight patients indicates the presence of only unmethylated products. Electrophoresis gel from nested MSP products detects methylation in Patients 3, 7, and 8. (B) Representative fluorescence spectra from two patients with differing methylation status. Significant acceptor (Cy5) emission at 670 nm is observed for patients with methylated PYCARD promoter (yellow trace). (C) Normalized FRET efficiencies (En) for 20 patients, conducted in a blinded fashion, indicate that Patients 3, 7, and 8 have methylation for PYCARD. An arbitrary En cut-off of 0.1 is used to determine positive methylation. All patients show unmethylated PYCARD product as well (data not shown).
Similar articles
- DNA methylation detection using MS-qFRET, a quantum dot-based nanoassay.
Bailey VJ, Keeley BP, Razavi CR, Griffiths E, Carraway HE, Wang TH. Bailey VJ, et al. Methods. 2010 Nov;52(3):237-41. doi: 10.1016/j.ymeth.2010.03.007. Epub 2010 Apr 1. Methods. 2010. PMID: 20362674 Review. - Influence of methylated p15 and p16 genes on clinicopathological features in colorectal cancer.
Ishiguro A, Takahata T, Saito M, Yoshiya G, Tamura Y, Sasaki M, Munakata A. Ishiguro A, et al. J Gastroenterol Hepatol. 2006 Aug;21(8):1334-9. doi: 10.1111/j.1440-1746.2006.04137.x. J Gastroenterol Hepatol. 2006. PMID: 16872319 - DNA hypermethylation of cell cycle (p15 and p16) and apoptotic (p14, p53, DAPK and TMS1) genes in peripheral blood of leukemia patients.
Bodoor K, Haddad Y, Alkhateeb A, Al-Abbadi A, Dowairi M, Magableh A, Bsoul N, Ghabkari A. Bodoor K, et al. Asian Pac J Cancer Prev. 2014;15(1):75-84. doi: 10.7314/apjcp.2014.15.1.75. Asian Pac J Cancer Prev. 2014. PMID: 24528084 - Methylation-specific loop-mediated isothermal amplification for detecting hypermethylated DNA in simplex and multiplex formats.
Zerilli F, Bonanno C, Shehi E, Amicarelli G, Adlerstein D, Makrigiorgos GM. Zerilli F, et al. Clin Chem. 2010 Aug;56(8):1287-96. doi: 10.1373/clinchem.2010.143545. Epub 2010 Jun 15. Clin Chem. 2010. PMID: 20551384 - The implications of heterogeneous DNA methylation for the accurate quantification of methylation.
Mikeska T, Candiloro IL, Dobrovic A. Mikeska T, et al. Epigenomics. 2010 Aug;2(4):561-73. doi: 10.2217/epi.10.32. Epigenomics. 2010. PMID: 22121974 Review.
Cited by
- Decoding circulating nucleic acids in human serum using microfluidic single molecule spectroscopy.
Liu KJ, Brock MV, Shih IeM, Wang TH. Liu KJ, et al. J Am Chem Soc. 2010 Apr 28;132(16):5793-8. doi: 10.1021/ja100342q. J Am Chem Soc. 2010. PMID: 20364832 Free PMC article. - Novel methylation biomarker panel for the early detection of pancreatic cancer.
Yi JM, Guzzetta AA, Bailey VJ, Downing SR, Van Neste L, Chiappinelli KB, Keeley BP, Stark A, Herrera A, Wolfgang C, Pappou EP, Iacobuzio-Donahue CA, Goggins MG, Herman JG, Wang TH, Baylin SB, Ahuja N. Yi JM, et al. Clin Cancer Res. 2013 Dec 1;19(23):6544-6555. doi: 10.1158/1078-0432.CCR-12-3224. Epub 2013 Oct 2. Clin Cancer Res. 2013. PMID: 24088737 Free PMC article. - A decade of exploring the cancer epigenome - biological and translational implications.
Baylin SB, Jones PA. Baylin SB, et al. Nat Rev Cancer. 2011 Sep 23;11(10):726-34. doi: 10.1038/nrc3130. Nat Rev Cancer. 2011. PMID: 21941284 Free PMC article. Review. - Quantum dot enabled molecular sensing and diagnostics.
Zhang Y, Wang TH. Zhang Y, et al. Theranostics. 2012;2(7):631-54. doi: 10.7150/thno.4308. Epub 2012 Jul 4. Theranostics. 2012. PMID: 22916072 Free PMC article. - High-resolution mapping studies of chromatin and gene regulatory elements.
Boyle AP, Furey TS. Boyle AP, et al. Epigenomics. 2009 Dec 1;1(2):319-329. doi: 10.2217/epi.09.29. Epigenomics. 2009. PMID: 20514362 Free PMC article.
References
- Belinsky SA, Liechty KC, Gentry FD, Wolf HJ, Rogers J, Vu K, Haney J, Kennedy TC, Hirsch FR, Miller Y, et al. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res. 2006;66:3338–3344. - PubMed
- Brandes JC, van Engeland M, Wouters KA, Weijenberg MP, Herman JG. CHFR promoter hypermethylation in colon cancer correlates with the microsatellite instability phenotype. Carcinogenesis. 2005;26:1152–1156. - PubMed
- Brock MV, Hooker CM, Ota-Machida E, Han Y, Guo M, Ames S, Glockner S, Piantadosi S, Gabrielson E, Pridham G, et al. DNA methylation markers and early recurrence in stage I lung cancer. N Engl J Med. 2008;358:1118–1128. - PubMed
- Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA058184/CA/NCI NIH HHS/United States
- R21 CA120742/CA/NCI NIH HHS/United States
- P50-CA058184-10/CA/NCI NIH HHS/United States
- R21-CA120742-01/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous