Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury - PubMed (original) (raw)
Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury
Drew L Sellers et al. J Neurosci. 2009.
Abstract
Progenitors that express NG2-proteoglycan are the predominant self-renewing cells within the CNS. NG2 progenitors replenish oligodendrocyte populations within the intact stem cell niche, and cycling NG2 cells are among the first cells to react to CNS insults. We investigated the role of NG2 progenitors after spinal cord injury and how bone morphogen protein signals remodel the progressive postinjury (PI) niche. Progeny labeled by an NG2-specific reporter virus undergo a coordinated shift in differentiation profile. NG2 progeny born 24 h PI produce scar-forming astrocytes and transient populations of novel phagocytic astrocytes shown to contain denatured myelin within cathepsin-D-labeled endosomes, but NG2 progenitors born 7 d PI differentiate into oligodendrocytes and express myelin on processes that wrap axons. Analysis of spinal cord mRNA shows a temporal shift in the niche transcriptome of ligands that affect PI remodeling and direct progenitor differentiation. We conclude that NG2 progeny are diverse lineages that obey progressive cues after trauma to replenish the injured niche.
Figures
Figure 1.
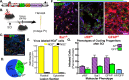
Cycling NG2+ cells produce multiple cell types after SCI. A, The NG2 promoter was used in a bicistronic retrovirus to drive the expression of EGFP, and AP (Alkaline Phos.) expression was controlled by the internal LTR. B–E, The NG2-reporter virus was used to track NG2 progenitors after injury via GFP expression (C) and labeled ∼33% of infected progenitors (D) with >95% of EGFP-expressing cells coexpressing NG2-proteoglycan (E). EGFP expression evaluated the phenotypes of cycling NG2+ progeny. F, Confocal microscopy was used to determine the molecular phenotypes of progeny derived from NG2 progenitors via colocalization of EGFP with Iba1 (microglia marker), vWF (blood-vasculature pericytes), and GFAP (glial marker). Scale bar, ∼10 μm. G, The virally labeled cell phenotypes were quantified as a function of AP or GFP expression (3024 cells) in adjacent serial sections stained with vWF, Iba1, and GFAP. Twenty-four hours PI, the majority of cycling NG2+ cells displays a reactive phenotype (GFAP+ or Iba1+), and NG2 progenitors comprise almost 50% (combined) of reactive phenotypes (AP+Iba1+ and NG2+Iba1+ vs AP+ and NG2+GFAP+; microglia and astrocytes, respectively). Error bars represent SEM.
Figure 2.
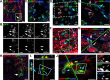
Progeny derived from NG2 progenitors participate in wound clearing. A, B, Confocal microscopy revealed a subset of cycling NG2+ cells that did not express GFAP (A, arrow) and possessed large vacuoles (∼5 μm) within the cell body (B, arrows; inset in A). C, Vacuolated EGFP+ cells colocalized with s100β immunofluorescence but did not express ED1 (asterisk). D, Adjacent serial sections show the vacuolated s100β+EGFP+ cells (cyan arrow) are a morphologically distinct population of phagocytic cell within the lesion, which are not Iba1+. E, An orthogonal view of a confocal _z_-stack shows that vacuolated s100β+EGFP+ cells (cyan arrows) do not label with other markers for phagocytic microglia (Iba1; magenta arrow). F, Immunofluorescence with antibodies against dMBP (MBP 81–92) revealed the s100β+ progeny (EGFP+), within the lesion epicenter, are a phagocytic phenotype and contained denature MBP within their cytoplasm. G, An orthogonal view, rotated about the _z_-axis (inset in F), clearly shows the MBP contained within numerous EGFP+ cell processes (arrows). H, The vacuolated s100β phenotypes do not persist outside the lesion epicenter where less denatured myelin is present (2 mm rostral; arrow) and are distinct from activated Iba+ microglia. I, Immunofluorescence of CathD shows colocalization (cyan arrows) of the endosome protein in cells that contain dMBP (yellow arrow). J, The rotated view of a confocal _z_-stack (inset in I) shows CathD localization on vacuoles and EGFP+ processes (cyan arrows) that flank dMBP. Inset, Monochrome breakouts show distinct localization of dMBP within an EGFP process decorated with CathD (asterisks, arrows). Scale bars, ∼10 μm.
Figure 3.
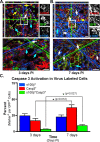
NG2+ phagocytes transition to become apoptotic 7 d after SCI. Immunofluorescence of virally labeled NG2+ cells, born 24 h PI, was quantified to determine whether s100β+EGFP+ cells (cyan arrows) become apoptotic, since the phenotype is not seen in cells born 7 d PI. A, B, Confocal images of tissue harvested from animals at 3 d (A) and 7 d (B) PI was imuunofluorescently stained with antibodies against Casp3 and s100β. Rotational views generated from each designated inset of serial sections show the morphology and colocalization of Casp3+ cells and showed a significant (*p = 0.027) increase in Casp3+s100β+EGFP+ cells (arrows) between 3 and 7 d PI (C; 3.65 ± 0.46% to 9.23 ± 1.49%, respectively). Overall, apoptotic cells (yellow arrow; Casp3+) increased significantly (p = 0.012) from 3 and 7 d PI (16.77 ± 3.6% to 36.97 ± 4.4%, respectively). Scale bars, 10 μm. Error bars indicate SEM.
Figure 4.
Progeny from cycling NG2+ cells show temporal shifts in differentiation profile after SCI. A, The NG2-reporter virus was used to label cycling NG2 progenitors at 24 h PI or 7 d PI. The phenotypes of the labeled cells were examined at 3 and 14 d PI. B, Fifty-three percent of the progeny derived from NG2 cells born 24 h PI produce astrocytes (GFAP+) compared with 24% APC+ immature oligodendrocytes. The direction of phenotype undergoes a significant temporal shift (***p < 0.001) to produce more oligodendrocytes (53%) and fewer (35%) when the progenitors are born 7 d after SCI and evaluated 14 d PI. Error bars represent SEM. C, The astrocyte progeny (born 24 h PI) are seen within the lesion epicenter expressing a gloitic phenotype (GFAP+ and CSPG+). D, A high-resolution _z_-stack (tilted orthogonal view in inset in C) shows distinct colocalization of GFAP with EGFP+ processes in cells born 24 h PI (arrows). Inset, Color channel-breakouts highlight the morphology and localization of CSPG on GFAP+ cells. E, Progeny derived from progenitors cycling at 7 d PI express MBP by 14 d PI. F, A high-resolution _z_-stack (tilted orthogonal view in inset in E) shows distinct colocalization of MBP with EGFP+ processes (arrows) in cells born 7 d PI that encircle NF+ profiles. Color channel-breakouts show an NF+ profile circumscribed by an EGFP+MBP+ process. Scale bars, 10 μm.
Figure 5.
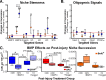
Temporal shifts in the expression of molecular cues and BMP ligand injection modulate gliogenesis in the PI niche. Real-time PCR on cDNA derived from injurious tissue (24 h or 7 d PI; orange and blue symbols, respectively) quantified the difference in expression of 17 genes. The fold change in gene expression is expressed as the difference in threshold cycle compared with GAPDH expression of experimental versus control values (24 h or 7d PI; circles and squares, respectively). A, BMP4 expression is elevated 2.5-fold at 24 h PI and continues to increase by 7 d PI (8.9-fold), which could influence the increase in early Id1 expression 24 h PI (up 2-fold). Whereas Id3 becomes elevated 3.6-fold by 7 d PI and Shh expression is suppressed 80% (0.24 of controls), Id2 did not change significantly. B, Factors known to be necessary for oligodendrocyte differentiation have reduced expression levels 24 h PI (Olig1, PDGFα) but return to control levels by 7 d PI to facilitate remyelination. *p = 0.05; **p < 0.01; †††p < 0.001 by t test. Error bars represent SEM. C, BMP signaling was modulated in SCI by direct injection of BMP4 (24 h PI) and noggin (7 d PI). Gliogenesis (GFAP+ and APC+ cells) was reduced significantly (p = 0.021) in BMP4-injected animals. noggin increased astrogliogenesis (GFAP+) but decreased overall numbers of oligodendrocytes (APC+) in animals injected 7 d PI. Cell proliferation was not affected by BMP4 or noggin (statistics determined by one-tailed t test; error bars represent SEM).
Figure 6.
NG2 progenitors respond to BMP4 when transplanted into a progressive niche. A, The NG2 promoter was used to drive the expression of CRE recombinase and infect spinal cord progenitors isolate from ROSA::GFP mice (26Sor cells). B, After infection, induced GFP expression was analyzed by activated fluorescence, and cells (26Sor* cells) were purified via FACS (EGFP high). C, mRNA isolated from 26Sor* cells confirmed CRE expression in cells that express NG2 and EGFP [shown with molecular weight markers (MW)]. D, Sor26* cells were amplified in cell culture and analyzed, via FACS, before transplantation into SCI. E, The stemness of 26Sor* cells was examined under differentiation conditions to produce the s100β+ astrocyte, CNPase+ oligodendrocyte, and Map2+ neuronal phenotypes. F, Hemisection-SCI animals received transplants of 26Sor* cells at 24 h and 7 d PI (with and without BMP4). The differentiation profile of transplanted 26Sor* was examined 14 d after transplantation (TXP). G, BMP4 increased the percentage GFAP+ 26Sor* cells (31.3%; H, I, cyan arrows), compared with naive controls (16.1%; J, K), to exceed the percentage of GFAP+ cells in animals that received transplants at 7 d PI (25.6%; ***p = 0.032). BMP4 did not alter the percentage of APC+ 26Sor* cells (yellow arrows) significantly (statistics determined by one-tailed t test). Rotated views of confocal _z_-plane stacks (I and K from H and J, respectively) showed increases in the proportions of GFAP colocalization (I) versus APC (K) on 26Sor* cells transplanted with BMP4 24 h PI. Monochrome breakouts (from insets) show distinct localization of GFAP and APC with EGFP+ processes (cyan and yellow arrows, respectively). Scale bars, ∼10 μm. Error bars represent SEM.
Similar articles
- Dependence of regenerated sensory axons on continuous neurotrophin-3 delivery.
Hou S, Nicholson L, van Niekerk E, Motsch M, Blesch A. Hou S, et al. J Neurosci. 2012 Sep 19;32(38):13206-20. doi: 10.1523/JNEUROSCI.5041-11.2012. J Neurosci. 2012. PMID: 22993437 Free PMC article. - NG2-proteoglycan-dependent contributions of oligodendrocyte progenitors and myeloid cells to myelin damage and repair.
Kucharova K, Stallcup WB. Kucharova K, et al. J Neuroinflammation. 2015 Sep 4;12:161. doi: 10.1186/s12974-015-0385-6. J Neuroinflammation. 2015. PMID: 26338007 Free PMC article. - Proliferating NG2-Cell-Dependent Angiogenesis and Scar Formation Alter Axon Growth and Functional Recovery After Spinal Cord Injury in Mice.
Hesp ZC, Yoseph RY, Suzuki R, Jukkola P, Wilson C, Nishiyama A, McTigue DM. Hesp ZC, et al. J Neurosci. 2018 Feb 7;38(6):1366-1382. doi: 10.1523/JNEUROSCI.3953-16.2017. Epub 2017 Dec 26. J Neurosci. 2018. PMID: 29279310 Free PMC article. - Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells.
Nishiyama A, Watanabe M, Yang Z, Bu J. Nishiyama A, et al. J Neurocytol. 2002 Jul-Aug;31(6-7):437-55. doi: 10.1023/a:1025783412651. J Neurocytol. 2002. PMID: 14501215 Review. - Instructive niches: environmental instructions that confound NG2 proteoglycan expression and the fate-restriction of CNS progenitors.
Sellers DL, Horner PJ. Sellers DL, et al. J Anat. 2005 Dec;207(6):727-34. doi: 10.1111/j.1469-7580.2005.00480.x. J Anat. 2005. PMID: 16367800 Free PMC article. Review.
Cited by
- Oligodendrocyte fate after spinal cord injury.
Almad A, Sahinkaya FR, McTigue DM. Almad A, et al. Neurotherapeutics. 2011 Apr;8(2):262-73. doi: 10.1007/s13311-011-0033-5. Neurotherapeutics. 2011. PMID: 21404073 Free PMC article. Review. - Understanding the NG2 Glial Scar after Spinal Cord Injury.
Hackett AR, Lee JK. Hackett AR, et al. Front Neurol. 2016 Nov 15;7:199. doi: 10.3389/fneur.2016.00199. eCollection 2016. Front Neurol. 2016. PMID: 27895617 Free PMC article. Review. - Intraventricular injections of mesenchymal stem cells activate endogenous functional remyelination in a chronic demyelinating murine model.
Cruz-Martinez P, González-Granero S, Molina-Navarro MM, Pacheco-Torres J, García-Verdugo JM, Geijo-Barrientos E, Jones J, Martinez S. Cruz-Martinez P, et al. Cell Death Dis. 2016 May 12;7(5):e2223. doi: 10.1038/cddis.2016.130. Cell Death Dis. 2016. PMID: 27171265 Free PMC article. - Beta-catenin signaling increases in proliferating NG2+ progenitors and astrocytes during post-traumatic gliogenesis in the adult brain.
White BD, Nathe RJ, Maris DO, Nguyen NK, Goodson JM, Moon RT, Horner PJ. White BD, et al. Stem Cells. 2010 Feb;28(2):297-307. doi: 10.1002/stem.268. Stem Cells. 2010. PMID: 19960516 Free PMC article.
References
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. - PubMed
- Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. - PubMed
- Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49:318–338. - PubMed
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous