Cdh1 regulates cell cycle through modulating the claspin/Chk1 and the Rb/E2F1 pathways - PubMed (original) (raw)
Cdh1 regulates cell cycle through modulating the claspin/Chk1 and the Rb/E2F1 pathways
Daming Gao et al. Mol Biol Cell. 2009 Jul.
Abstract
APC/Cdh1 is a major cell cycle regulator and its function has been implicated in DNA damage repair; however, its exact role remains unclear. Using affinity purification coupled with mass spectrometry, we identified Claspin as a novel Cdh1-interacting protein and further demonstrated that Claspin is a novel Cdh1 ubiquitin substrate. As a result, inactivation of Cdh1 leads to activation of the Claspin/Chk1 pathway. Previously, we demonstrated that Rb interacts with Cdh1 to influence its ability to degrade Skp2. Here, we report that Cdh1 reciprocally regulates the Rb pathway through competing with E2F1 to bind the hypophosphorylated form of Rb. Although inactivation of Cdh1 in HeLa cells, with defective p53/Rb pathways, led to premature S phase entry, acute depletion of Cdh1 in primary human fibroblasts resulted in premature senescence. Acute loss of many other major tumor suppressors, including PTEN and VHL, also induces premature senescence in a p53- or Rb-dependent manner. Similarly, we showed that inactivation of the p53/Rb pathways by overexpression of SV40 LT-antigen partially reversed Cdh1 depletion-induced growth arrest. Therefore, loss of Cdh1 is only beneficial to cells with abnormal p53 and Rb pathways, which helps explain why Cdh1 loss is not frequently found in many tumors.
Figures
Figure 1.
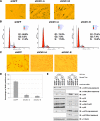
Identification of Claspin as a novel Cdh1-interacting protein. (A) Immunoblot analysis of the T98G cell lines infected with control (EV) or pBabe-retroviral vector expressing HA.Flag-tagged Cdh1. (B) Cellular lysates were prepared from both cell lines, and subjected to HA immunoprecipitation. After extensive washes, the recovered proteins were separated by SDS-PAGE and stained with Gel-code blue reagent. (C) Illustration of the peptide sequences identified in the Cdh1-IP by mass spectrometry analysis. (D) Cell lysates were prepared from the two generated T98G cells, and after immunoprecipitation with HA antibody, immunoblot analyses with indicated antibody were performed to detect their ability to interact with ectopically expressed Cdh1 protein. (E) 293T cells were transiently transfected with various constructs as indicated. Forty-eight hours after transfection, cell lysates were recovered, and HA immunoprecipitation was performed. The immunoprecipitates were denatured in SDS-containing sample buffer and separated by SDS-PAGE before immunoblot analysis with indicated antibodies. (F) HA-Claspin and various Myc-tagged Cdh1 constructs were cotransfected into 293T cells. Forty-eight hours after transfection, cell lysates were recovered, and HA immunoprecipitation was performed. The immunoprecipitates were denatured in SDS-containing sample buffer and separated by SDS-PAGE before immunoblot analysis with HA and Myc antibodies.
Figure 2.
Cdh1 controls Claspin stability. (A and B) Depletion of Cdh1 by siRNA treatments leads to significant induction of Claspin protein in both asynchronized (A) and synchronized (B) HeLa cells. (C) Immunoblot analysis of the U2OS cells, engineered to overexpress Myc-Cdh1 after removal of tetracycline to induce Cdh1, with indicated antibodies. (D–E) Myc-Claspin and HA-Cdh1 or HA-Cdc20 constructs were cotransfected into HeLa cells as indicated, and the differences in Claspin expression levels were detected by anti-Myc immunoblot analysis. MG132 was added in D as indicated to block the proteasomal degradation pathway.
Figure 3.
Claspin contains a novel degron at its N-terminus that governs its destruction by Cdh1. (A) Summary of the nonoverlapping Claspin fragments and their interaction with and destruction by Cdh1. (B) Illustration of the various Claspin mutants to map the location of the C-box. (C and D) Immunoblot analysis of HeLa cells transfected with the indicated Myc-Claspin plasmid, synchronized by growth in nocodazole, and then released for the indicated periods of time. (E) Sequence alignment of the novel degron sequence among different species.
Figure 4.
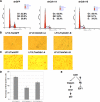
Depletion of Cdh1 leads to unscheduled activation of Chk-1 activity in the G1 phase. (A) Depletion of Cdh1 by siRNA treatments leads to a significant induction of Claspin protein and activation of the Chk1/p53 pathway in asynchronized U2OS cells. (B) HeLa cells were transfected with indicated siRNA oligos, and 6 h after transfection, cells were synchronized with nocodazole. Eighteen hours later, cells were released back into the G1 phase, and at the various indicated time points, cells were lysed for immunoblot analysis using the indicated antibodies. (C) HeLa cells were transfected with the indicated siRNA oligos; 6 h after transfection, cells were synchronized with nocodazole. Eighteen hours later, cells were released back into the G1 phase. At various indicated time points, cells were pulsed with BrdU for 30 min, and afterward immunohistochemistry staining was performed with anti-BrdU antibody to detect the percentage of BrdU-positive cells. (D) Illustration of the proposed model by which Cdh1 could potentially activate Chk1 and p53 activity by controlling Claspin destruction.
Figure 5.
Acute loss of Cdh1 expression resulted in the onset of premature senescence in primary human fibroblasts. (A–C) Primary human lung fibroblast cells were infected with the indicated lentiviral shRNA constructs. Twenty-four hours after infection, cells were selected with 1 μg/ml puromycin to eliminate the noninfected cells. Seven days after puromycin selection, cells were fixed and stained for senescence-associated β-Gal activity (A). Six days after puromycin selection, cells were fixed by 70% ethanol and the cell cycle distribution was examined by FACS analysis (B). Six days after puromycin selection, cells were incubated with 1 μg/μl BrdU and 100 μg/μl uridine for 6 h before being fixed with cold methanol and subjected to immunohistochemical analysis using anti-BrdU antibody (C). (D) Quantification of the percentage of BrdU-positive cells in C. (E) Immunoblot analysis of primary human lung fibroblasts (LF1) and SV40 LT-antigen expressing LF1 (LF1/LT) cells infected with the indicated shRNA lentiviral vectors. Twenty-four hours after infection, cells were selected with 1 μg/ml puromycin to eliminate the noninfected cells. Whole-cell lysates were collected at the indicated times after infection.
Figure 6.
Overexpression of LT-ag partially blocked the premature senescence phenotype induced by depletion of Cdh1 in primary human fibroblasts. (A–C) LF1/LT cells were infected with the indicated lentiviral shRNA constructs. Twenty-four hours after infection, cells were selected with 1 μg/ml puromycin to eliminate the noninfected cells. Six days after puromycin selection, cells were fixed by 70% ethanol, and the cell cycle distribution was examined by FACS analysis (A). Seven days after puromycin selection, cells were fixed and stained for senescence-associated β-Gal activity (B). Six days after puromycin selection, cells were incubated with 1 μg/μl BrdU and 100 μg/μl uridine for 6 h before being fixed with cold methanol and subjected to immunohistochemical analysis using anti-BrdU antibody (C). (D) Quantification of the percentage of BrdU-positive cells in C. (E) Illustration of the proposed model by which Cdh1 could potentially regulate the activity of both the Claspin/Chk1/p53 and Rb/E2F1 pathways.
Figure 7.
Cdh1 regulates the Rb/E2F1 pathway. (A) Immunoblot analysis of primary human lung fibroblasts and primary human foreskin fibroblasts infected with indicated shRNA lentiviral vectors. Twenty-four hours after infection, cells were selected with 1 μg/ml puromycin to eliminate the noninfected cells. Whole-cell lysates were harvested 6 d after puromycin selection. (B) Immunoblot analysis of the U2OS cells that were engineered to overexpress Myc-Cdh1 after removal of tetracycline with indicated antibodies. (C–E) Autoradiograms showing recovery of 35S-labeled Rb protein bound to GST-Cdh1 fusion proteins. Where indicated, in vitro–translated Rb protein was incubated with phosphatase (C) or cyclinA/Cdk2 kinase (D) before the pulldown assays. (E) Increased amount of in vitro–translated E2F1 protein was added to the binding reactions (3, 9, and 27 μl, as indicated by the triangles). (F) Autoradiograms showing recovery of 35S-labeled Rb protein bound to GST-E2F1 fusion proteins. Where indicated, increased amount of in vitro-translated Cdh1 protein was added to the binding reactions (3, 9, and 27 μl, as indicated by the triangles). (G) Illustration of the proposed model by which Cdh1 could potentially regulate the abundance of E2F1 through the Rb protein.
Similar articles
- Cyclin A/Cdk2 regulates Cdh1 and claspin during late S/G2 phase of the cell cycle.
Oakes V, Wang W, Harrington B, Lee WJ, Beamish H, Chia KM, Pinder A, Goto H, Inagaki M, Pavey S, Gabrielli B. Oakes V, et al. Cell Cycle. 2014;13(20):3302-11. doi: 10.4161/15384101.2014.949111. Cell Cycle. 2014. PMID: 25485510 Free PMC article. - Adenovirus E1A oncoprotein liberates c-Myc activity to promote cell proliferation through abating Bin1 expression via an Rb/E2F1-dependent mechanism.
Kinney EL, Tanida S, Rodrigue AA, Johnson JK, Tompkins VS, Sakamuro D. Kinney EL, et al. J Cell Physiol. 2008 Sep;216(3):621-31. doi: 10.1002/jcp.21437. J Cell Physiol. 2008. PMID: 18348166 - Epithelial cell-derived periostin functions as a tumor suppressor in gastric cancer through stabilizing p53 and E-cadherin proteins via the Rb/E2F1/p14ARF/Mdm2 signaling pathway.
Lv H, Liu R, Fu J, Yang Q, Shi J, Chen P, Ji M, Shi B, Hou P. Lv H, et al. Cell Cycle. 2014;13(18):2962-74. doi: 10.4161/15384101.2014.947203. Cell Cycle. 2014. PMID: 25486483 Free PMC article. - Claspin, a regulator of Chk1 in DNA replication stress pathway.
Chini CC, Chen J. Chini CC, et al. DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1033-7. doi: 10.1016/j.dnarep.2004.03.001. DNA Repair (Amst). 2004. PMID: 15279790 Review. - Claspin - checkpoint adaptor and DNA replication factor.
Smits VAJ, Cabrera E, Freire R, Gillespie DA. Smits VAJ, et al. FEBS J. 2019 Feb;286(3):441-455. doi: 10.1111/febs.14594. Epub 2018 Jun 29. FEBS J. 2019. PMID: 29931808 Review.
Cited by
- mTOR drives its own activation via SCF(βTrCP)-dependent degradation of the mTOR inhibitor DEPTOR.
Gao D, Inuzuka H, Tan MK, Fukushima H, Locasale JW, Liu P, Wan L, Zhai B, Chin YR, Shaik S, Lyssiotis CA, Gygi SP, Toker A, Cantley LC, Asara JM, Harper JW, Wei W. Gao D, et al. Mol Cell. 2011 Oct 21;44(2):290-303. doi: 10.1016/j.molcel.2011.08.030. Mol Cell. 2011. PMID: 22017875 Free PMC article. - Cyclin A/Cdk2 regulates Cdh1 and claspin during late S/G2 phase of the cell cycle.
Oakes V, Wang W, Harrington B, Lee WJ, Beamish H, Chia KM, Pinder A, Goto H, Inagaki M, Pavey S, Gabrielli B. Oakes V, et al. Cell Cycle. 2014;13(20):3302-11. doi: 10.4161/15384101.2014.949111. Cell Cycle. 2014. PMID: 25485510 Free PMC article. - Regulation of E2F1 Transcription Factor by Ubiquitin Conjugation.
Dubrez L. Dubrez L. Int J Mol Sci. 2017 Oct 19;18(10):2188. doi: 10.3390/ijms18102188. Int J Mol Sci. 2017. PMID: 29048367 Free PMC article. Review. - Phosphorylation of EZH2 by AMPK Suppresses PRC2 Methyltransferase Activity and Oncogenic Function.
Wan L, Xu K, Wei Y, Zhang J, Han T, Fry C, Zhang Z, Wang YV, Huang L, Yuan M, Xia W, Chang WC, Huang WC, Liu CL, Chang YC, Liu J, Wu Y, Jin VX, Dai X, Guo J, Liu J, Jiang S, Li J, Asara JM, Brown M, Hung MC, Wei W. Wan L, et al. Mol Cell. 2018 Jan 18;69(2):279-291.e5. doi: 10.1016/j.molcel.2017.12.024. Mol Cell. 2018. PMID: 29351847 Free PMC article. - Functional characterization of Anaphase Promoting Complex/Cyclosome (APC/C) E3 ubiquitin ligases in tumorigenesis.
Zhang J, Wan L, Dai X, Sun Y, Wei W. Zhang J, et al. Biochim Biophys Acta. 2014 Apr;1845(2):277-93. doi: 10.1016/j.bbcan.2014.02.001. Epub 2014 Feb 22. Biochim Biophys Acta. 2014. PMID: 24569229 Free PMC article. Review.
References
- Agami R., Bernards R. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell. 2000;102:55–66. - PubMed
- Bartkova J., et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. - PubMed
- Bashir T., Dorrello N. V., Amador V., Guardavaccaro D., Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–193. - PubMed
- Bennett L. N., Clarke P. R. Regulation of Claspin degradation by the ubiquitin-proteosome pathway during the cell cycle and in response to ATR-dependent checkpoint activation. FEBS Lett. 2006;580:4176–4181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM053203/GM/NIGMS NIH HHS/United States
- R01 GM089763/GM/NIGMS NIH HHS/United States
- R01 GM53203/GM/NIGMS NIH HHS/United States
- R01 GM89763/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous