NG2+/Olig2+ cells are the major cycle-related cell population of the adult human normal brain - PubMed (original) (raw)
NG2+/Olig2+ cells are the major cycle-related cell population of the adult human normal brain
Sameh Geha et al. Brain Pathol. 2010 Mar.
Abstract
A persistent cycling cell population in the normal adult human brain is well established. Neural stem cells or neural progenitors have been identified in the subventricular zone and the dentate gyrus subgranular layer (SGL), two areas of persistent neurogenesis. Cycling cells in other human normal brain areas, however, remains to be established. Here, we determined the distribution and identity of these cells in the cortex, the white matter and the hippocampal formation of adult patients with and without chronic temporal lobe epilepsy using immunohistochemistry for the cell cycle markers Ki-67 (Mib-1) and minichromosome maintenance protein 2. Rare proliferative neuronal precursors expressing the neuronal antigen neuronal nuclei were restricted to the SGL. In contrast, the oligodendrocyte progenitor cell markers Olig2 and the surface antigen NG2 were expressed by the vast majority of cycling cells scattered throughout the cortex and white matter of both control and epileptic patients. Most of these cycling cells were in early G1 phase, and were significantly more numerous in epileptic than in non-epileptic patients. These results provide evidence for a persistent gliogenesis in the human cortex and white matter that is enhanced in an epileptic environment.
Figures
Figure 1
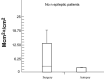
Comparative Mcm‐2 immunodetection in tissues derived from the surgical and autopsy control groups. Fewer Mcm2 labeled cells are detected in the autopsy control group (mean fixation time: 3, 2 months) than in the surgical control group (mean fixation time: 18 h). Abbreviations: Mcm2 = minichromosome maintenance protein 2.
Figure 2
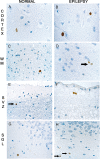
Cycling cells are dispersed in the cortex and the white matter of the control (A,C,E,G) and epileptic brains (B,D,F,H). Mib‐1‐positive nuclei are restricted to round monomorphous regular nuclei, in the cortex (A,B) or in the white matter (C,D, arrow). In the autopsy control group, rare Mcm2‐positive cells are detected in the lateral ventricle SVZ (E, arrow) as well as in the SGL (G). In the SVZ of epileptic patients, pairs of Mcm‐2‐positive nuclei are visualized (F) and some single cells are present in the SGL (H, arrows). Magnification in A,C,D,E,G,H: ×600; B,F: ×1000. Scale bar = 10 µm. Abbreviations: Mcm2 = minichromosome maintenance protein 2; Mib‐1 = Ki‐67; SGL = subgranular layer; SVZ = subventricular zone; WM = white matter.
Figure 3
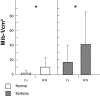
Comparative data show that Mcm2‐positive cells/cm2 are consistently more abundant than Mib‐1‐positive cells/cm2 in the surgical control group (P = 0.006) and in the external temporal lobe (ETL) (P = 0.001) and the hippocampal formation (P = 0.002) of epileptic patients. Abbreviations: Mcm2 = minichromosome maintenance protein 2; Mib‐1 = Ki‐67.
Figure 4
The densities of Mib‐1‐positive cells are not statistically different in the white matter (WM) and the cortex (Cx) of either the surgical control group or the epileptic group. Abbreviations: Mib‐1 = Ki‐67.
Figure 5
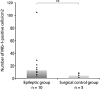
Increased numbers of Mib‐1‐positive cells/cm2 in the external temporal parenchyma of epileptic patients as compared with the surgical control group (P = 0.017).
Figure 6
Double immunostainings show that cycling cells co‐express Olig2 or NG2 in the surgical control brain. A,B. No Mib‐1‐positive cells (red, arrow) co‐express GFAP (brown) (A) or CNPase (brown) (B). C. All Mib‐1‐positive cells (red) co‐localized with non‐endothelial NG2‐positive cells (brown). D–F. Confocal microscopy of double immuno‐fluorescence stainings of Olig2 (red, D) and Mib‐1 (green, E) reveal that all Mib‐1‐positive cells co‐express Olig2 (F, overlay of D and E). Magnification in A,B,D,E and F: ×600; C: ×1000. Scale bar = 20 µm. Abbreviations: GFAP = glial fibrillary associated protein; CNPase = 2′ 3′‐cyclic nucleotide 3′‐phosphodiesterase; Mib‐1 = Ki‐67.
Figure 7
Double immunostainings revealed that cycling cells do not share markers of the astrocytes, microglia, mature oligodendrocytes or neurons, except in the SGL of the epileptic patients. No Mib‐1‐positive cell (red) co‐express GFAP (A,B), vimentin (C,D), KP1 (E,F), CNPase (G), MAP‐2 (H), nestin (I) or calretinin (J). Confocal microscopy of double immuno‐fluorescence stainings of NeuN (red, K) and Mib‐1 (green, L) in cells of the subgranular layer (overlay, M). Magnification = ×400 in A–J, ×600 in K–M. Scale bar = 7 µm. Abbreviations: GFAP = glial fibrillary associated protein; CNPase = 2′ 3′‐cyclic nucleotide 3′‐phosphodiesterase; Mib‐1 = Ki‐67; MAP2 = microtubule associate protein 2; NeuN = neuronal nuclei; WM = white matter.
Figure 8
NG2 expressing cells are dispersed in the white matter (A,B,E,I) and the cortex (C,D) in epileptic patients and presented a multipolar appearance. Double immunostainings revealed that all NG2‐positive cells (brown, B,D) co‐expressed Olig2 (red, B,D) in the white matter as well as in the cortex, sometimes in a perineuronal satellitosis location (D, arrow). A unique NG2‐positive cell was in mitosis (brown, E). Double immuno‐fluorescence stainings showed that in epileptic patients cycling cells correspond to Olig2 and NG2‐expressing cells. Confocal microscopy revealed that all Mib‐1‐positive cells (green, G,K) co‐expressed the oligodendrocyte progenitor cells markers NG2 (red, F) or Olig2 (red, J). H,L. overlay respectively of F and G and J and K. Double chromogenic immunostaining of NG2/ Mib‐1 confirm that some paired of fine ramified NG2‐positive cells (brown) co‐expressed Mib‐1 (red) (I). Magnification = ×400 in A–C,E,I; =×600 in D,F–H,J–L, scale bar = 30 µm.
Similar articles
- PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice.
Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. Rivers LE, et al. Nat Neurosci. 2008 Dec;11(12):1392-401. doi: 10.1038/nn.2220. Epub 2008 Oct 8. Nat Neurosci. 2008. PMID: 18849983 Free PMC article. - Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum.
Chung SH, Guo F, Jiang P, Pleasure DE, Deng W. Chung SH, et al. Cell Death Dis. 2013 Mar 14;4(3):e546. doi: 10.1038/cddis.2013.74. Cell Death Dis. 2013. PMID: 23492777 Free PMC article. - Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex.
Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M. Dimou L, et al. J Neurosci. 2008 Oct 8;28(41):10434-42. doi: 10.1523/JNEUROSCI.2831-08.2008. J Neurosci. 2008. PMID: 18842903 Free PMC article. - Searching for oligodendrocyte precursors for cell replacement therapies.
Sypecka J. Sypecka J. Acta Neurobiol Exp (Wars). 2011;71(1):94-102. doi: 10.55782/ane-2011-1826. Acta Neurobiol Exp (Wars). 2011. PMID: 21499330 Review.
Cited by
- Hippocampal neurogenesis in adult primates: a systematic review.
Elliott T, Liu KY, Hazan J, Wilson J, Vallipuram H, Jones K, Mahmood J, Gitlin-Leigh G, Howard R. Elliott T, et al. Mol Psychiatry. 2024 Nov 18. doi: 10.1038/s41380-024-02815-y. Online ahead of print. Mol Psychiatry. 2024. PMID: 39558003 - Integrated electrophysiological and genomic profiles of single cells reveal spiking tumor cells in human glioma.
Curry RN, Ma Q, McDonald MF, Ko Y, Srivastava S, Chin PS, He P, Lozzi B, Athukuri P, Jing J, Wang S, Harmanci AO, Arenkiel B, Jiang X, Deneen B, Rao G, Serin Harmanci A. Curry RN, et al. Cancer Cell. 2024 Oct 14;42(10):1713-1728.e6. doi: 10.1016/j.ccell.2024.08.009. Epub 2024 Sep 5. Cancer Cell. 2024. PMID: 39241781 - Neurogenic potential of NG2 in neurotrauma: a systematic review.
Rigo YR, Benvenutti R, Portela LV, Strogulski NR. Rigo YR, et al. Neural Regen Res. 2024 Dec 1;19(12):2673-2683. doi: 10.4103/NRR.NRR-D-23-01031. Epub 2024 Mar 1. Neural Regen Res. 2024. PMID: 38595286 Free PMC article. - Neuron-oligodendroglial interactions in health and malignant disease.
Taylor KR, Monje M. Taylor KR, et al. Nat Rev Neurosci. 2023 Dec;24(12):733-746. doi: 10.1038/s41583-023-00744-3. Epub 2023 Oct 19. Nat Rev Neurosci. 2023. PMID: 37857838 Free PMC article. Review. - Senescence, brain inflammation, and oligomeric tau drive cognitive decline in Alzheimer's disease: Evidence from clinical and preclinical studies.
Gaikwad S, Senapati S, Haque MA, Kayed R. Gaikwad S, et al. Alzheimers Dement. 2024 Jan;20(1):709-727. doi: 10.1002/alz.13490. Epub 2023 Oct 9. Alzheimers Dement. 2024. PMID: 37814508 Free PMC article. Review.
References
- Arsenijevic Y, Villemure JG, Brunet JF, Bloch JJ, Deglon N, Kostic C et al (2001) Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Exp Neurol 170:48–62. - PubMed
- Baron W, Metz B, Bansal R, Hoekstra D, De Vries H (2000) PDGF and FGF‐2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol Cell Neurosci 15:314–329. - PubMed
- Blumcke I, Schewe JC, Normann S, Brustle O, Schramm J, Elger CE, Wiestler OD (2001) Increase of nestin‐immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early‐onset temporal lobe epilepsy. Hippocampus 11:311– 321. - PubMed
- Butt AM, Duncan A, Hornby MF, Kirvell SL, Hunter A, Levine JM, Berry M (1999) Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia 26:84–91. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous