Selective killing of cancer cells by suppression of geminin activity - PubMed (original) (raw)
Selective killing of cancer cells by suppression of geminin activity
Wenge Zhu et al. Cancer Res. 2009.
Abstract
Eukaryotic cells normally restrict genome duplication to once per cell division. In metazoa, re-replication of DNA during a single S phase seems to be prevented solely by suppressing CDT1 activity, a protein required for loading the replicative MCM DNA helicase. However, siRNA suppression of geminin (a specific inhibitor of CDT1) arrested proliferation only of cells derived from cancers by inducing DNA re-replication and DNA damage that spontaneously triggered apoptosis. None of these effects were detected either in cells derived from normal human tissues or in cells immortalized by a viral oncogene. To induce these effects in noncancer cells required suppression of both geminin and cyclin A, another cell cycle regulator. Therefore, initiating DNA replication in some cancer cells is limited solely by regulating the level of CDT1 activity with geminin, whereas noncancer cells contain additional safeguards that prevent DNA re-replication. These results show that inhibition of geminin activity could be used to selectively kill cancer cells without harming other cells.
Figures
Figure 1
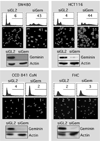
Depletion of geminin induced DNA re-replication in colorectal cancer cells (SW480, HCT116), but not in normal colon cells (CCD 841 CoN, FHC). The indicated cells were transfected with siRNA against either firefly luciferase (siGL2) or human geminin (siGem). At 48 hours post-transfection, cells were harvested and stained either with propidium iodide to quantify their DNA content by fluorescence activated flow cytometry (FACS) analysis, or with DAPI to visualize their nuclei by fluorescence microscope. The percentage of cells is given with greater than 4N DNA content (FACS profiles) or with nuclei whose diameter is greater than twice that of nuclei in siGL2 treated cells. Geminin and actin proteins were detected by Western immuno-blotting.
Figure 2
Geminin depletion suppressed cancer cell proliferation and induced apoptosis. (A) Colon carcinoma HCT116 cells were transfected with either siGem (■) or siGL2 (□),harvested at indicated times post-transfection, and the total number of cells recorded. (B) Cells treated as in (A) were subjected to Western immuno-blotting for geminin and actin. (C) The fraction of cells in (A) that remained attached to the dish with giant nuclei was determined by fluorescent microscopy. (D) Total cells (attached plus unattached) in (A) were analyzed by FACS. Percentage with less than 2N DNA content, and the percentage with greater than 4N DNA content are indicated.
Figure 3
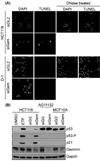
Only cells that re-replicated their DNA experienced genotoxic stress and apoptosis. (A) Colon carcinoma HCT116 cells were transfected with either siGL2 or siGem. At 4 days post-transfection, cells not attached to the dish were isolated by centrifugation and subjected to the TUNEL assay. HCT116 cells treated with DNase I provided a TUNEL positive control. Normal skin D1 cells were treated likewise. (B) The indicated cells were treated with either siGL2 or siGem as in Fig. 1, and then subjected to Western immuno-blotting for the indicated protein (p53-P is p53 phosphorylated on serine 15). To demonstrate the effects of genotoxic stress, p53+/+ HCT116 cells were treated with 3 µM etoposide (ETP) for 16 hours in parallel with HCT116 cells treated with the same buffered solvent (DMSO). Longer exposures revealed p53 in HCT116 cells, and shorter exposures confirmed that p53 levels in AF11132 and MCF10A cells remained unchanged.
Figure 4
Depletion of geminin did not suppress proliferation of normal cells and did not induce apoptosis. (A) The total number of normal skin D1 cells transfected with either siGL2 (□) or siGem (■) at the indicated times post-transfection. (B) Cells treated as in (A) were subjected to Western immuno-blotting for geminin and actin. ‘Control’ indicates an unidentified protein that cross-reacted with anti-geminin antibody. (C) Cells in (A) were analyzed by FACS, as in Fig. 1. Percentage of cells with >4N DNA is shown. (D) The indicated cells were transfected with either siGem or siGL2. Six days later the total number of cells was counted, and the ratio of cells surviving siGem transfection relative to those surviving siGL2 transfection was determined.
Figure 5
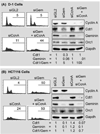
Normal cells prevent DNA re-replication through multiple convergent pathways that are dependent on both geminin and cyclin A. Normal skin D1 cells (A) and colon carcinoma HCT116 cells (B) were transfected either with siGL2, with siGem, with siCcnA, or with both siGem and siCcnA. Cells were harvested at two days post-transfection and subjected to FACS analysis to determine the fraction of cells with greater than 4N DNA content. The same cells were subjected to Western immuno-blotting to determine the relative levels of cyclin A, CDT1, geminin, and Orc1. Protein levels were quantified using Multi Gauge software (Fuji Film). Cdt1 and geminin protein levels were normalized relative to Gapdh, and then to either Cdt1 or geminin in siGl2 treated cells.
Similar articles
- Characterization of conserved arginine residues on Cdt1 that affect licensing activity and interaction with Geminin or Mcm complex.
You Z, Ode KL, Shindo M, Takisawa H, Masai H. You Z, et al. Cell Cycle. 2016 May 2;15(9):1213-26. doi: 10.1080/15384101.2015.1106652. Cell Cycle. 2016. PMID: 26940553 Free PMC article. - Cdt1 and geminin are down-regulated upon cell cycle exit and are over-expressed in cancer-derived cell lines.
Xouri G, Lygerou Z, Nishitani H, Pachnis V, Nurse P, Taraviras S. Xouri G, et al. Eur J Biochem. 2004 Aug;271(16):3368-78. doi: 10.1111/j.1432-1033.2004.04271.x. Eur J Biochem. 2004. PMID: 15291814 - Cdt1 and geminin: role during cell cycle progression and DNA damage in higher eukaryotes.
Tada S. Tada S. Front Biosci. 2007 Jan 1;12:1629-41. doi: 10.2741/2175. Front Biosci. 2007. PMID: 17127409 Review. - Proteolysis of DNA replication licensing factor Cdt1 in S-phase is performed independently of geminin through its N-terminal region.
Nishitani H, Lygerou Z, Nishimoto T. Nishitani H, et al. J Biol Chem. 2004 Jul 16;279(29):30807-16. doi: 10.1074/jbc.M312644200. Epub 2004 May 11. J Biol Chem. 2004. PMID: 15138268 - Cdt1 and Geminin in cancer: markers or triggers of malignant transformation?
Petropoulou C, Kotantaki P, Karamitros D, Taraviras S. Petropoulou C, et al. Front Biosci. 2008 May 1;13:4485-94. doi: 10.2741/3018. Front Biosci. 2008. PMID: 18508524 Review.
Cited by
- A high-throughput screening identifies MCM chromatin loading inhibitors targeting cells with increased replication origins.
Falbo L, Técher H, Sannino V, Robusto M, Fagà G, Pezzimenti F, Romeo F, Colombo LG, Vultaggio S, Fancelli D, Monzani S, Cecatiello V, Pasqualato S, Varasi M, Mercurio C, Costanzo V. Falbo L, et al. iScience. 2024 Jul 22;27(8):110567. doi: 10.1016/j.isci.2024.110567. eCollection 2024 Aug 16. iScience. 2024. PMID: 39184446 Free PMC article. - Quantity and quality of minichromosome maintenance protein complexes couple replication licensing to genome integrity.
Yadav AK, Polasek-Sedlackova H. Yadav AK, et al. Commun Biol. 2024 Feb 9;7(1):167. doi: 10.1038/s42003-024-05855-w. Commun Biol. 2024. PMID: 38336851 Free PMC article. Review. - Polymolecular botanical drug of Orthosiphon stamineus extract (C5OSEW5050ESA) as a complementary therapy to overcome gemcitabine resistance in pancreatic cancer cells.
Yehya AHS, Asif M, Abdul Majid AMS, Oon CE. Yehya AHS, et al. J Tradit Complement Med. 2022 Oct 14;13(1):39-50. doi: 10.1016/j.jtcme.2022.10.002. eCollection 2023 Jan. J Tradit Complement Med. 2022. PMID: 36685076 Free PMC article. - Lysophosphatidic acid suppresses apoptosis of high-grade serous ovarian cancer cells by inducing autophagy activity and promotes cell-cycle progression via EGFR-PI3K/Aurora-AThr288-geminin dual signaling pathways.
Zhao H, Jia P, Nanding K, Wu M, Bai X, Morigen M, Fan L. Zhao H, et al. Front Pharmacol. 2022 Dec 19;13:1046269. doi: 10.3389/fphar.2022.1046269. eCollection 2022. Front Pharmacol. 2022. PMID: 36601056 Free PMC article. - High expression of GMNN predicts malignant progression and poor prognosis in ACC.
Zhao X, Zhang X, Shao S, Yang Q, Shen C, Yang X, Jiao W, Liu J, Wang Y. Zhao X, et al. Eur J Med Res. 2022 Dec 20;27(1):301. doi: 10.1186/s40001-022-00950-2. Eur J Med Res. 2022. PMID: 36539849 Free PMC article.
References
- Kelly TJ, Stillman B. Duplication of DNA in Eukaryotic Cells. In: DePamphilis ML, editor. DNA Replication and Human Disease. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2006. pp. 1–30.
- DePamphilis ML, Blow JJ, Ghosh S, Saha T, Noguchi K, Vassilev A. Regulating the licensing of DNA replication origins in metazoa. Curr Opin Cell Biol. 2006;18:231–239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials