AGTR1 overexpression defines a subset of breast cancer and confers sensitivity to losartan, an AGTR1 antagonist - PubMed (original) (raw)
. 2009 Jun 23;106(25):10284-9.
doi: 10.1073/pnas.0900351106. Epub 2009 Jun 1.
Bushra Ateeq, Qi Cao, Scott A Tomlins, Rohit Mehra, Bharathi Laxman, Shanker Kalyana-Sundaram, Robert J Lonigro, Beth E Helgeson, Mahaveer S Bhojani, Alnawaz Rehemtulla, Celina G Kleer, Daniel F Hayes, Peter C Lucas, Sooryanarayana Varambally, Arul M Chinnaiyan
Affiliations
- PMID: 19487683
- PMCID: PMC2689309
- DOI: 10.1073/pnas.0900351106
AGTR1 overexpression defines a subset of breast cancer and confers sensitivity to losartan, an AGTR1 antagonist
Daniel R Rhodes et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Breast cancer patients have benefited from the use of targeted therapies directed at specific molecular alterations. To identify additional opportunities for targeted therapy, we searched for genes with marked overexpression in subsets of tumors across a panel of breast cancer profiling studies comprising 3,200 microarray experiments. In addition to prioritizing ERBB2, we found AGTR1, the angiotensin II receptor type I, to be markedly overexpressed in 10-20% of breast cancer cases across multiple independent patient cohorts. Validation experiments confirmed that AGTR1 is highly overexpressed, in several cases more than 100-fold. AGTR1 overexpression was restricted to estrogen receptor-positive tumors and was mutually exclusive with ERBB2 overexpression across all samples. Ectopic overexpression of AGTR1 in primary mammary epithelial cells, combined with angiotensin II stimulation, led to a highly invasive phenotype that was attenuated by the AGTR1 antagonist losartan. Similarly, losartan reduced tumor growth by 30% in AGTR1-positive breast cancer xenografts. Taken together, these observations indicate that marked AGTR1 overexpression defines a subpopulation of ER-positive, ERBB2-negative breast cancer that may benefit from targeted therapy with AGTR1 antagonists, such as losartan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
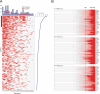
MetaCOPA analysis of breast cancer gene expression data. (A) MetaCOPA map. Each column in the map represents a breast cancer gene expression dataset. The numbers at the base of the map correspond to dataset details (
Table S1
). Each row indicates a gene. A red cell indicates that the gene was deemed to have an outlier expression profile in the respective dataset because it scored in the top 1% of COPA values at 1 of 3 percentile cutoffs. The line graph along the y axis indicates the P value for a gene based on the number of datasets in which the gene was deemed an outlier. A total of 158 genes were called outliers in a significant fraction of datasets (P < 1E-5). The bar graph indicates the number of samples in the respective datasets and the contribution of the dataset to the meta-analysis. The black bar on the left of the map indicates the top 25 meta-outliers, which are detailed in B for 3 datasets marked with an asterisk. (B) Heatmaps of COPA-normalized values for top-scoring meta-outliers across 3 highly contributory datasets: Miller et al. (26), Hess et al. (27), and Wang et al. (28). Genes are ranked by their MetaCOPA P values. For each gene, samples are ordered from left to right by their COPA-normalized expression values. Highest intensity of red indicates a COPA-normalized value of 6 or greater. White indicates a value of zero or less.
Fig. 2.
AGTR1 outlier expression in breast cancer. (A) AGTR1 expression profile in the Perou et al. (29) cDNA microarray dataset (n = 55). (B) In the same dataset, AGTR1 expression vs. ERBB2 expression. (C) AGTR1 expression profile in the van de Vijver et al. (30) oligonucleotide dataset, segregated by ER status (n = 295). (D) AGTR1 expression vs. ERBB2 expression in the same dataset. (E) AGTR1 expression by quantitative RT-PCR in formalin-fixed, paraffin-embedded tissue. Expression of AGTR1 was assessed in 3 normal breast tissue specimens, 36 primary breast tumor specimens, and 9 metastatic breast cancer specimens. Expression levels were normalized to GAPDH expression and then scaled by the median AGTR/GADPH ratio.
Fig. 3.
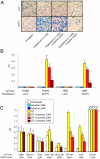
Copy number analysis of the AGTR1 locus. (A) A schematic of probes used for FISH analysis. (B) Representative image from FISH analysis. Left is taken from a representative negative case. Middle and Right are images from a representative case with definitive copy number gain of AGTR1. Red signal is the AGTR1 locus probe, and green signal is the probe near the chromosome 3 centromere. (C) Association of AGTR1 overexpression with copy number gain. Three expression bins were defined based on AGTR1/GAPDH ratios: low (<1.0), moderate (1.0–2.0), and high (>2.0).
Fig. 4.
AGTR1 overexpression and analysis of angiotensin II (AT) and losartan effects on cell invasion. (A) Matrigel invasion assays of H16N2 cells infected with adenovirus expressing AGTR1 or LacZ. Cells were cultured in serum-free media and were pretreated with and without AT and losartan. Similar results were observed for HME cells. (B) Colorimetry readout of invasion assays from transfection experiments. LacZ- or AGTR1-expressing adenovirus was infected into H16N2 and HME immortalized mammary epithelial cells, and cells were treated with or without 1 μM AT and losartan. Because of absent baseline invasion, the optical density (OD) measurements were background subtracted, and values below 0.01 were set to 0.01. (C) Colorimetry readout of invasion assays from a panel of cancer cell lines. Seven breast cancer cell lines and a prostate cancer cell line, DU145, were examined for invasion after treatment with or without 1 μM AT and losartan. AGTR1 expression levels are indicated and were obtained from published microarray data and qRT-PCR analysis (
Fig. S7
). The quantification of invasion was done as described in B.
Fig. 5.
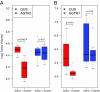
Effect of losartan treatment on AGTR1- or GUS-overexpressing MCF7 cell xenografts. Female BALB/C nu/nu mice were implanted with 2.5 × 106 stable MCF7 cells overexpressing AGTR1 or GUS resuspended in 100 μL of saline with 20% Matrigel into the mammary fat pad of anesthetized mice. Mice from both groups: MCF7-AGTR1 or MCF7-GUS (n = 10 for each group) were treated every day with losartan (90 mg/kg body weight) or vehicle control. All animals were monitored at weekly intervals for tumor growth, and tumor sizes were recorded using the formula (π/6) (L × _W_2), where L = length of tumor and W = width. Box plots of log2 tumor volumes are shown. P values from 2-sided Student's t tests indicate statistical significance. (A) Xenograft tumor size at 2 weeks. (B) Xenograft tumor size at 8 weeks.
Similar articles
- Overexpression of angiotensin II type 1 receptor in breast cancer cells induces epithelial-mesenchymal transition and promotes tumor growth and angiogenesis.
Oh E, Kim JY, Cho Y, An H, Lee N, Jo H, Ban C, Seo JH. Oh E, et al. Biochim Biophys Acta. 2016 Jun;1863(6 Pt A):1071-81. doi: 10.1016/j.bbamcr.2016.03.010. Epub 2016 Mar 11. Biochim Biophys Acta. 2016. PMID: 26975580 - AGTR1 as a therapeutic target in ER-positive and ERBB2-negative breast cancer cases.
Ateeq B, Tomlins SA, Chinnaiyan AM. Ateeq B, et al. Cell Cycle. 2009 Dec;8(23):3794-5. doi: 10.4161/cc.8.23.9976. Cell Cycle. 2009. PMID: 19934656 Free PMC article. No abstract available. - Dual targeting of angiotensin receptors (AGTR1 and AGTR2) in epithelial ovarian carcinoma.
Park YA, Choi CH, Do IG, Song SY, Lee JK, Cho YJ, Choi JJ, Jeon HK, Ryu JY, Lee YY, Kim TJ, Bae DS, Lee JW, Kim BG. Park YA, et al. Gynecol Oncol. 2014 Oct;135(1):108-17. doi: 10.1016/j.ygyno.2014.06.031. Epub 2014 Jul 9. Gynecol Oncol. 2014. PMID: 25014541 - Losartan in diabetic nephropathy.
Perico N, Ruggenenti P, Remuzzi G. Perico N, et al. Expert Rev Cardiovasc Ther. 2004 Jul;2(4):473-83. doi: 10.1586/14779072.2.4.473. Expert Rev Cardiovasc Ther. 2004. PMID: 15225108 Review. - Role and therapeutic potential of G-protein coupled receptors in breast cancer progression and metastases.
Singh A, Nunes JJ, Ateeq B. Singh A, et al. Eur J Pharmacol. 2015 Sep 15;763(Pt B):178-83. doi: 10.1016/j.ejphar.2015.05.011. Epub 2015 May 14. Eur J Pharmacol. 2015. PMID: 25981295 Free PMC article. Review.
Cited by
- Inhalation delivery of Telmisartan enhances intratumoral distribution of nanoparticles in lung cancer models.
Godugu C, Patel AR, Doddapaneni R, Marepally S, Jackson T, Singh M. Godugu C, et al. J Control Release. 2013 Nov 28;172(1):86-95. doi: 10.1016/j.jconrel.2013.06.036. Epub 2013 Jul 7. J Control Release. 2013. PMID: 23838154 Free PMC article. - Alterations in Gene Expression of Components of the Renin-Angiotensin System and Its Related Enzymes in Lung Cancer.
Goldstein B, Trivedi M, Speth RC. Goldstein B, et al. Lung Cancer Int. 2017;2017:6914976. doi: 10.1155/2017/6914976. Epub 2017 Jul 16. Lung Cancer Int. 2017. PMID: 28791183 Free PMC article. - MALT1 Is a Targetable Driver of Epithelial-to-Mesenchymal Transition in Claudin-Low, Triple-Negative Breast Cancer.
Lee JL, Ekambaram P, Carleton NM, Hu D, Klei LR, Cai Z, Myers MI, Hubel NE, Covic L, Agnihotri S, Krappmann D, Bornancin F, Lee AV, Oesterreich S, McAllister-Lucas LM, Lucas PC. Lee JL, et al. Mol Cancer Res. 2022 Mar 1;20(3):373-386. doi: 10.1158/1541-7786.MCR-21-0208. Mol Cancer Res. 2022. PMID: 34753803 Free PMC article. - AGTR1 promoter hypermethylation in lung squamous cell carcinoma but not in lung adenocarcinoma.
Chen R, Hong Q, Jiang J, Chen X, Jiang Z, Wang J, Liu S, Duan S, Shi S. Chen R, et al. Oncol Lett. 2017 Oct;14(4):4989-4994. doi: 10.3892/ol.2017.6824. Epub 2017 Aug 25. Oncol Lett. 2017. PMID: 29085512 Free PMC article. - 125I-Angiotensin 1-7 binds to a different site than angiotensin 1-7 in tissue membrane preparations.
Stoyell-Conti FF, Itty S, Abraham C, Rigatto K, West CA, Speth RC. Stoyell-Conti FF, et al. Endocrine. 2021 May;72(2):529-538. doi: 10.1007/s12020-020-02572-2. Epub 2021 Jan 7. Endocrine. 2021. PMID: 33415576
References
- King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985;229:974–976. - PubMed
- Slamon DJ, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
- Di Fiore PP, et al. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987;237:178–182. - PubMed
- Piccart-Gebhart MJ, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA046592/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- PC040517/PC/NCI NIH HHS/United States
- UO1 CA111275-01/CA/NCI NIH HHS/United States
- U01 CA111275/CA/NCI NIH HHS/United States
- PC020322/PC/NCI NIH HHS/United States
- 5P30 CA46592/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous