Frequent loss of cystatin E/M expression implicated in the progression of prostate cancer - PubMed (original) (raw)
Frequent loss of cystatin E/M expression implicated in the progression of prostate cancer
S M Pulukuri et al. Oncogene. 2009.
Abstract
Cystatin E/M (CST6) is a natural inhibitor of lysosomal cysteine proteases. Recent studies have shown that experimental manipulation of CST6 expression alters the metastatic behavior of human breast cancer cells. However, the association of CST6 with prostate cancer invasion and progression remains unclear. Here, we show that CST6 is robustly expressed in normal human prostate epithelium, whereas its expression is downregulated in metastatic prostate cell lines and prostate tumor tissues. Treatment of metastatic prostate cell lines with the histone deacetylase inhibitor trichostatin A resulted in significant induction of CST6 mRNA levels and increased CST6 protein expression, indicating that epigenetic silencing may play a role in the loss of CST6 expression observed in prostate cancer. CST6 overexpression in human prostate cancer cells significantly reduced in vitro cell proliferation and matrigel invasion. Furthermore, the results from a bioluminescence tumor/metastasis model showed that the overexpression of CST6 significantly inhibits tumor growth and the incidence of lung metastasis. These results suggest that the downregulation of the CST6 gene is associated with promoter histone modifications and that this association plays an important role in prostate cancer progression during the invasive and metastatic stages of the disease.
Figures
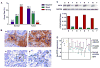
Figure 1. Expression of CST6 in human prostate tissue samples
(A) Compared with normal prostate tissue, the overall expression level of CST6 in the prostate cancer tissue was significantly lower (P < 0.001). Tissue sections were prepared from formalin-fixed, paraffin-embedded specimens of normal and tumor human prostate tissues. Immunostaining was carried out using a specific anti-human CST6 antibody. CST6 expression in normal human prostate tissue (32 cases) and prostate cancer (42 cases) was analyzed. (B) Representative immunostaining photographs were taken at different magnifications: a, normal human prostate showing CST6 in epithelial cells; b, high-power view of (a) showing membrane staining of CST6; c, lack of CST6 staining in prostate cancer tissues; d, high-power view of (c) showing lack of CST6 expression in prostate cancer tissues (p < 0.001); T, tumor cells; N, normal prostate epithelial cells. (C) Total cell extracts were further prepared from four-paired normal prostate (N) and prostate tumor tissue (T) specimens. The 14-kDa unglycosylated and 17-kDa glycosylated forms of CST6 protein expression was determined by immunoblot analysis. MDA-MB-231 cells (C) that express CST6 were used as a positive control for the specificity of the antibody. The level of CST6 protein expression was significantly lower in tumor tissue than in normal tissue, which was indicated by the ratio of CST6/GAPDH (bottom). (D) Quantitative mRNA expression of CST6 in human prostate tumor (green) compared to normal adjacent tissue of the same individual (red, connected by a line). RNA was isolated and reverse transcribed and SYBR Green real-time PCR carried out with CST6-specific primers. Significant downregulation of CST6 expression is observed in tumor tissues as compared to respective normal adjacent tissues.
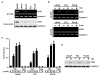
Figure 2. Expression of CST6 in human prostate epithelial and cancer cells, and effect of trichostatin A (TSA)
(A) RT-PCR (top) and immunoblot (bottom) analysis of CST6 in prostate cancer cell lines. Cystatin C was used as loading control for RNA and protein analysis. (B) CST6 mRNA levels in control and TSA-treated LNCaP, PC3 and PC3-M cells were analyzed by semiquantitative RT-PCR (top). CST6 mRNA levels (223-bp amplicon) in control and 5-aza-treated LNCaP, PC3 and PC3-M cells were analyzed by semiquantitative RT-PCR (bottom). Cystatin C mRNA was amplified as a loading control and expression standard. (C) Real-time RT-PCR analysis of CST6 mRNA expression in LNCaP, PC3 and PC3-M cells treated with TSA or 5-aza. RNA was isolated and reverse transcribed and SYBR Green real-time PCR carried out with CST6-specific primers. Columns, mean of three independent experiments; bars, SD. (D)Immunoblot analysis of CST6 protein expression in LNCaP, PC3 and PC3-M cells with or without TSA treatment. The CST6 protein expression was determined by immunoblot analysis. Cystatin C was used as a loading control to check for equal loading of the gel.
Figure 3. TSA induces accumulation of acetylated histones H3 and H4 in chromatin associated with the CST6 gene
(A) Nuclear extracts were isolated from control and TSA-treated LNCaP, PC3 and PC3-M cells, and immunoblot analysis was performed using anti-acetyl histone H3, anti-acetyl histone H4, and histone H3 antibodies. Histone H3 was utilized as a loading control. (B) Densitometric analysis of immunoblots in (A) from control and TSA-treated cell lines. Data are normalized to H3, averaged, and expressed as percentage of control (CTL=1). (C) Schematic representation of the CST6 promoter region and the location of primers used for PCR amplification in the ChIP assay. Bent arrow, transcriptional start site (+). (D) Chromatin fragments from LNCaP, PC3 and PC3-M cells cultured with (+) or without (−) TSA for 20 h were immunoprecipitated with antibody to acetylated (Ac) histones H3 and H4 or control normal rabbit serum (NRS). PCR primers for the CST6 and β-actin promoters were used to amplify the DNA isolated from the immunoprecipitated chromatin as described in Materials and Methods. Note the CST6 promoter specific primers amplified PCR fragment of 157-bp.
Figure 4. Overexpression of CST6 reduces proliferation and invasion of PC3 prostate cancer cells
(A) RT-PCR (top) and immunoblot (bottom) analyses of PC3 cells stably transfected with mock, CST6 expression vector or empty vector. CST6 expression at both the mRNA and protein levels was detected only in PC3 cells stably expressing CST6 clones (CST6-4, CST6-12, CST6-13, CST6-18), but not from empty vector clone or mock. Cystatin C was used as loading control for RNA and protein analysis. (B) Proliferation of PC3 cells stably transfected with either CST6 expression vector or controls (mock or empty vector-transfected cells) was revealed by MTT assay. Each bar represents triplicate analyses of mean ± S.D. where the significant difference from controls is represented by an asterisk (*, p < 0.05). (C) Comparison of the in vitro invasive potentials of PC3 cells stably transfected with mock, CST6 expression vector, or empty vector. A representative number of invading cells through the matrigel were counted under the microscope in five random fields at a 200× magnification. Each bar represents the mean ±S.D. of five fields counted. Significant difference from controls (mock or siControl-transfected cells) is indicated by an asterisk (*, p < 0.05). (D) Representative invasion photographs from PC3 cells stably transfected with mock, CST6 expression vector, or empty vector as described in (C). (E) mRNA (left) and protein (right) levels of cathepsins in PC3 cells stably transfected with mock, CST6 expression vector or empty vector. The number under each band is expressed as a percentage of mock control, normalized by the corresponding cystatin C level. Cystatin C was used as loading control for RNA and protein analysis.
Figure 5. Downregulation of CST6 induces proliferation and invasion via upregulation of cathepsin B
(A) mRNA (left) and protein (right) levels of cathepsins in RWPE1 cells transfected with mock, scramble control siRNA (siCTL) or CST6 siRNA (siCST6). Presence of the 223-bp CST6 mRNA amplicon and two forms of CST6 protein is evident. The number under each band is expressed as a percentage of mock control, normalized by the corresponding cystatin C level. Cystatin C was used as loading control for RNA and protein analysis. (B) Proliferation of RWPE1 cells transfected with mock, siCTL or siCST6 was revealed by MTT assay. Each bar represents triplicate analyses of mean ± S.D. where the significant difference from controls is represented by an asterisk (*, p < 0.05). (C) Comparison of the in vitro invasive potentials of RWPE1 cells transfected with mock, siCTL or siCST6. A representative number of invading cells through the matrigel were counted under the microscope in five random fields at a 200× magnification. Each bar represents the mean ±S.D. of five fields counted. Significant difference from controls (mock or siControl−transfected cells) is indicated by an asterisk (*, p < 0.05). (D) Representative invasion photographs from RWPE1 cells transfected with mock, siCTL or siCST6 as described in (C).
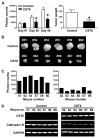
Figure 6. CST6 overexpression inhibits prostate tumor growth and metastasis in nude mice
(A) PC3 cells stably expressing luciferase reporter with either empty vector (control) or CST6 expression vector were injected into mouse prostate, and luciferase activity was recorded for each mouse. Photon counts of orthotopic prostate tumors on days 10, 20, and 40 (left). Comparison of dissected prostate tumors in (A) from mice 40 days after cell implantation. Each bar represents the mean tumor weight ± S.D. of six animals per group. Significant difference from control group is indicated by an asterisk (*, p < 0.05) (right). (B) Prostate tumors in (A), from mice 40 days after cell implantation, were excised and photographed. (C) Quantification of luciferase activities from either each control mouse (left) or each _CST6_-expressing mouse (right). (D) RNA samples extracted from prostate tumors (6 animals/group) were analyzed using RT-PCR for CST6 and cathepsin B expression levels. GAPDH mRNA was amplified as a loading control and expression standard.
Similar articles
- DNA methylation-dependent silencing of CST6 in human breast cancer cell lines.
Rivenbark AG, Jones WD, Coleman WB. Rivenbark AG, et al. Lab Invest. 2006 Dec;86(12):1233-42. doi: 10.1038/labinvest.3700485. Epub 2006 Oct 16. Lab Invest. 2006. PMID: 17043665 - Invasion suppressor cystatin E/M (CST6): high-level cell type-specific expression in normal brain and epigenetic silencing in gliomas.
Qiu J, Ai L, Ramachandran C, Yao B, Gopalakrishnan S, Fields CR, Delmas AL, Dyer LM, Melnick SJ, Yachnis AT, Schwartz PH, Fine HA, Brown KD, Robertson KD. Qiu J, et al. Lab Invest. 2008 Sep;88(9):910-25. doi: 10.1038/labinvest.2008.66. Epub 2008 Jul 7. Lab Invest. 2008. PMID: 18607344 Free PMC article. - TBX2 represses CST6 resulting in uncontrolled legumain activity to sustain breast cancer proliferation: a novel cancer-selective target pathway with therapeutic opportunities.
D'Costa ZC, Higgins C, Ong CW, Irwin GW, Boyle D, McArt DG, McCloskey K, Buckley NE, Crawford NT, Thiagarajan L, Murray JT, Kennedy RD, Mulligan KA, Harkin DP, Waugh DJ, Scott CJ, Salto-Tellez M, Williams R, Mullan PB. D'Costa ZC, et al. Oncotarget. 2014 Mar 30;5(6):1609-20. doi: 10.18632/oncotarget.1707. Oncotarget. 2014. PMID: 24742492 Free PMC article. - Modeling prostate cancer in mice: limitations and opportunities.
Hensley PJ, Kyprianou N. Hensley PJ, et al. J Androl. 2012 Mar-Apr;33(2):133-44. doi: 10.2164/jandrol.111.013987. Epub 2011 Jun 16. J Androl. 2012. PMID: 21680808 Free PMC article. Review.
Cited by
- Structural and functional analysis of cystatin E reveals enzymologically relevant dimer and amyloid fibril states.
Dall E, Hollerweger JC, Dahms SO, Cui H, Häussermann K, Brandstetter H. Dall E, et al. J Biol Chem. 2018 Aug 24;293(34):13151-13165. doi: 10.1074/jbc.RA118.002154. Epub 2018 Jul 2. J Biol Chem. 2018. PMID: 29967063 Free PMC article. - The Role of Cysteine Cathepsins in Cancer Progression and Drug Resistance.
Rudzińska M, Parodi A, Soond SM, Vinarov AZ, Korolev DO, Morozov AO, Daglioglu C, Tutar Y, Zamyatnin AA Jr. Rudzińska M, et al. Int J Mol Sci. 2019 Jul 23;20(14):3602. doi: 10.3390/ijms20143602. Int J Mol Sci. 2019. PMID: 31340550 Free PMC article. Review. - Investigation of the clinical significance and prospective molecular mechanisms of cystatin genes in patients with hepatitis B virus‑related hepatocellular carcinoma.
Zhou X, Wang X, Huang K, Liao X, Yang C, Yu T, Liu J, Han C, Zhu G, Su H, Qin W, Han Q, Liu Z, Huang J, Gong Y, Ye X, Peng T. Zhou X, et al. Oncol Rep. 2019 Jul;42(1):189-201. doi: 10.3892/or.2019.7154. Epub 2019 May 9. Oncol Rep. 2019. PMID: 31115549 Free PMC article. - A closed-tube methylation-sensitive high resolution melting assay (MS-HRMA) for the semi-quantitative determination of CST6 promoter methylation in clinical samples.
Dimitrakopoulos L, Vorkas PA, Georgoulias V, Lianidou ES. Dimitrakopoulos L, et al. BMC Cancer. 2012 Oct 22;12:486. doi: 10.1186/1471-2407-12-486. BMC Cancer. 2012. PMID: 23088560 Free PMC article. - Proteolytic cleavage and truncation of NDRG1 in human prostate cancer cells, but not normal prostate epithelial cells.
Ghalayini MK, Dong Q, Richardson DR, Assinder SJ. Ghalayini MK, et al. Biosci Rep. 2013 Jun 11;33(3):e00042. doi: 10.1042/BSR20130042. Biosci Rep. 2013. PMID: 23634903 Free PMC article.
References
- Abrahamson M. Cystatins. Methods Enzymol. 1994;244:685–700. - PubMed
- Ai L, Kim WJ, Kim TY, Fields CR, Massoll NA, Robertson KD, et al. Epigenetic silencing of the tumor suppressor cystatin M occurs during breast cancer progression. Cancer Res. 2006;66:7899–7909. - PubMed
- Alvarez-Fernandez M, Barrett AJ, Gerhartz B, Dando PM, Ni J, Abrahamson M. Inhibition of mammalian legumain by some cystatins is due to a novel second reactive site. J Biol Chem. 1999;274:19195–19203. - PubMed
- Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene. 2003;22:6524–6536. - PubMed
- Brunner N, Pyke C, Hansen CH, Romer J, Grondahl-Hansen J, Dano K. Urokinase plasminogen activator (uPA) and its type 1 inhibitor (PAI-1): regulators of proteolysis during cancer invasion and prognostic parameters in breast cancer. Cancer Treat Res. 1994;71:299–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS061835/NS/NINDS NIH HHS/United States
- R01 CA075557-11/CA/NCI NIH HHS/United States
- R01 NS057529/NS/NINDS NIH HHS/United States
- R01 CA138409/CA/NCI NIH HHS/United States
- R01 CA116708-03/CA/NCI NIH HHS/United States
- CA138409/CA/NCI NIH HHS/United States
- CA95058/CA/NCI NIH HHS/United States
- R01 CA092393-04/CA/NCI NIH HHS/United States
- R01 CA075557-10/CA/NCI NIH HHS/United States
- R01 CA116708/CA/NCI NIH HHS/United States
- R01 NS047699/NS/NINDS NIH HHS/United States
- R01 CA095058/CA/NCI NIH HHS/United States
- R01 CA092393/CA/NCI NIH HHS/United States
- R01 CA116708-04/CA/NCI NIH HHS/United States
- R01 NS057529-03/NS/NINDS NIH HHS/United States
- R01 CA095058-05/CA/NCI NIH HHS/United States
- NS47699/NS/NINDS NIH HHS/United States
- R01 CA075557/CA/NCI NIH HHS/United States
- CA75557/CA/NCI NIH HHS/United States
- R01 NS061835-01/NS/NINDS NIH HHS/United States
- R01 CA092393-05/CA/NCI NIH HHS/United States
- NS57529/NS/NINDS NIH HHS/United States
- R01 NS047699-05/NS/NINDS NIH HHS/United States
- CA116708/CA/NCI NIH HHS/United States
- R01 CA138409-01A1/CA/NCI NIH HHS/United States
- CA92393/CA/NCI NIH HHS/United States
- R01 CA095058-04/CA/NCI NIH HHS/United States
- NS61835/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous