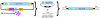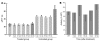Nanocapsule-delivered Sleeping Beauty mediates therapeutic Factor VIII expression in liver sinusoidal endothelial cells of hemophilia A mice - PubMed (original) (raw)
. 2009 Jul;119(7):2086-99.
doi: 10.1172/JCI34332. Epub 2009 Jun 8.
Affiliations
- PMID: 19509468
- PMCID: PMC2701853
- DOI: 10.1172/JCI34332
Nanocapsule-delivered Sleeping Beauty mediates therapeutic Factor VIII expression in liver sinusoidal endothelial cells of hemophilia A mice
Betsy T Kren et al. J Clin Invest. 2009 Jul.
Abstract
Liver sinusoidal endothelial cells are a major endogenous source of Factor VIII (FVIII), lack of which causes the human congenital bleeding disorder hemophilia A. Despite extensive efforts, gene therapy using viral vectors has shown little success in clinical hemophilia trials. Here we achieved cell type-specific gene targeting using hyaluronan- and asialoorosomucoid-coated nanocapsules, generated using dispersion atomization, to direct genes to liver sinusoidal endothelial cells and hepatocytes, respectively. To highlight the therapeutic potential of this approach, we encapsulated Sleeping Beauty transposon expressing the B domain-deleted canine FVIII in cis with Sleeping Beauty transposase in hyaluronan nanocapsules and injected them intravenously into hemophilia A mice. The treated mice exhibited activated partial thromboplastin times that were comparable to those of wild-type mice at 5 and 50 weeks and substantially shorter than those of untreated controls at the same time points. Further, plasma FVIII activity in the treated hemophilia A mice was nearly identical to that in wild-type mice through 50 weeks, while untreated hemophilia A mice exhibited no detectable FVIII activity. Thus, Sleeping Beauty transposon targeted to liver sinusoidal endothelial cells provided long-term expression of FVIII, without apparent antibody formation, and improved the phenotype of hemophilia A mice.
Figures
Figure 1. Size analysis and DNase protection of plasmids encapsulated by dispersion atomization.
(A) Schematic of the s50 capsule showing the overall structure and composition. The percentage of each constituent in the final formulated nanocapsule is indicated in parentheses. PEI, 25-kDa polyethylenimine. (B–E) Atomic force micrographs of HA-encapsulated pSV40:Alb-_lac_Z (B); ASOR-encapsulated pSV40:Ear-_lac_Z (C); ASOR-encapsulated cis _SB_-Tn/CAGGS-BΔcFVIII (D); and HA-encapsulated cis _SB_-Tn/CAGGS-BΔcFVIII (E) prepared as described in Methods. The size of the ASOR (C and D) and HA (B and E) nanocapsules with plasmids was determined by AFM image analysis using data collected in the tapping mode. Scale bar: 100 nm. Transmission electron micrographs of negatively stained ASOR-encapsulated pSV40:Ear-_lac_Z (F) and HA-encapsulated pSV40:Alb-_lac_Z (G). Scale bar: 50 nm. (H) DNase resistance of plasmid DNA in ASOR or HA nanocapsules. Plasmid DNA (1 μg) with or without encapsulation was subjected to DNase treatment and the DNA recovered as outlined in Methods. A 0.5-μg aliquot of DNA was separated on a 0.7% agarose gel and the plasmids visualized by ethidium bromide staining and UV light. Lane 1, pSV40:Ear-_lac_Z untreated; lane 2, pSV40:Alb-_lac_Z untreated; lane 3, pSV40:Ear-_lac_Z; lane 4, pSV40:Alb-_lac_Z; lane 5, pSV40:Ear-_lac_Z in ASOR nanocapsules; and lane 6, pSV40:Alb-_lac_Z in HA nanocapsules; lanes 3–6, treated. M, 1-kb ladder (Invitrogen).
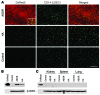
Figure 2. Delivery of nanoencapsulated DsRed2 _SB_-Tns in vivo to either hepatocytes or LSECs.
Eight-week-old mice were administered 100 μg of the encapsulated cis pT2/DsRed2 Tns via tail vein injection and sacrificed 1 week after injection. Expression of DsRed2 targeted to hepatocytes with ASOR or to LSECs with HA was visualized by confocal microscopy. LSECs were identified by anti-CD14 Ab, a marker specific for the discontinuous endothelial cells in the liver, and a Cy5-labeled secondary Ab. The confocal micrographs (A) show Cy5-labeled LSECs (green) with DsRed2 fluorescence (red). The targeting ligand for the nanocapsules and the relevant protein are indicated at left and above, respectively. The merged images (right) of the DsRed2 and CD14 micrographs demonstrate colocalization (yellow) of fluorescence when HA was used as the targeting ligand. The inset in the top left panel shows SYTOX green–stained nuclei (green) of hepatocytes expressing DsRed2; original magnification, ×60. Scale bar, 50 μm. (B) Western blot analysis of 100 μg total liver protein extracts from control mice and mice treated with the ASOR or HA nanocapsules shown in A. The proteins were detected by ECL as described in Methods. The treatment group is indicated above the lanes. (C) Western blot analysis of 100 μg total protein extracts from kidney, spleen, and lung to determine nonspecific uptake of nanocapsules. The β-actin lanes for loading controls are shown below. Control, mice treated with HA nanocapsules not containing DsRed2-expressing _SB_-Tns; DsRed2, purified recombinant DsRed2 protein.
Figure 3. Cell and promoter specificity of nanocapsule targeting in vivo.
Eight-week-old male and female mice were administered 100 μg of the encapsulated plasmid targeted to liver using ASOR or HA via tail vein injection and sacrificed 1 week after injection. (A–D) β-gal expression in 6-μm liver cryosections was visualized by immunohistochemical staining using rabbit anti–β-gal and a mouse cocktail specific for microvessels (78). The micrographs show the red color of the secondary anti-rabbit Qdot 565 conjugate in hepatocytes expressing β-gal using either the hepatocyte-specific SV40:Alb promoter (B) or the constitutive SV40:Ear (C) delivered to hepatocytes via ASOR. In contrast, when LSECs were targeted by HA nanocapsules, no detectable expression of β-gal using the SV40:Alb promoter occurred (A). (D) Background staining observed in control liver from vehicle-injected mice. The green FITC-labeled microvessels are shown to provide a reference for β-gal expression. The targeting ligand and plasmid are indicated above and below the respective panels. (E–J) Expression of β-gal in animals receiving pcDNA3.1 delivered using ASOR (E and H), HA (F and I), or vehicle control (G and J) determined by IHC against the Xpress epitope tag. The positive Xpress signal (green) gives an obvious hepatocyte (E) and LSEC (F) expression pattern, while no staining is observed in the negative vehicle control (G). The merged panels below show the β-gal expression with the blue nuclei counterstain TO-PRO-3. The targeting ligand is indicated above and the Ab and nuclear stain on the right.
Figure 4. PCR analysis of DNA from livers of male and female mice injected with ASOR or HA nanocapsules.
(A) PCR amplification of the prokaryotic β-gal CDS. Lane 1, DNA from vehicle-injected control; lanes 2 and 3, ASOR nanocapsules containing pSV40:Alb-_lac_Z and pSV40:Ear-_lac_Z, respectively; lane 4, HA nanocapsules containing pSV40:Alb-_lac_Z. (B) RT-PCR of RNA isolated from livers of mice treated with HA (lane 1) or ASOR (lanes 2 and 3) nanocapsules with the _lac_Z gene controlled by SV40:Alb (lanes 1 and 2) or SV40:Ear promoter (lane 3) or RNA from a vehicle-treated control (lane 4). (C) Representative gel of amplicons produced as in A using 5, 1, 0.5, 0.1, 0.05, and 0.01 pg (lanes 1–6, respectively) of pSV40:Alb as template. The size and location of the predicted amplicons are indicated at left (A) and at right (B and C). M, 2-log DNA ladder, with bands in 100-bp increments. (D) Densitometric data from 4 independent dilution and amplifications using the DNA template concentrations listed in C. The data points are mean ± SD, with the best-fit curve equation and correlation coefficient shown. (E) Examination of other tissues by PCR. PCR amplification of _lac_Z using the same primers as for A and DNA from kidney, lung, and spleen (lane 1–3, respectively) from a female mouse treated with pSV40:Alb-_lac_Z encapsulated using ASOR and vehicle control spleen DNA (lane 4). Kidney, lung, spleen, and testis (lanes 5–8) from a male treated with pSV40:Alb-_lac_Z encapsulated using HA. Lanes 9–16, apoB control PCR using the same template DNA and presented in the same order. The apoB amplicon and location of the 345-bp _lac_Z product are shown. (F) RT-PCR assessment of biodistribution. RT-PCR detection of the _lac_Z mRNA in RNA from the liver of mice (left panel) treated with ASOR-nanoencapsulated pcDNA 3.1/His/_lac_Z (lane 1) or from vehicle-treated controls (lane 2). RT-PCR of RNA (right panel) isolated from control kidney (lane 1) and kidney, lung, spleen, and testis (lanes 2–5, respectively) from a male treated with ASOR-encapsulated pSV40:Ear; kidney, lung, and spleen (lanes 6–8) from a female treated with ASOR-encapsulated pcDNA3.1/His/_lac_Z. The location of the 345-bp _lac_Z PCR product is indicated.
Figure 5. Schematic of FVIII _SB_-Tn.
_SB_-Tn in cis showing the relative size and position of the 2 eukaryotic expression cassettes (left). IR/DRs (black arrows) flank the CAGGS promoter–driven (C) BΔcFVIII transgene that utilizes the SV40 poly(A) signal from the original BΔcFVIII vector. External to the Tn IR/DRs, the vector backbone carries the required _SB_10 transposase expressed from the eukaryotic initiation factor 4A1 promoter (eIF). After transfection, the obligate _SB_10 transposase (pink) is expressed and binds to the IR/DRs and excises the Tn from the plasmid vector. The transposase cuts and pastes the Tn into random genomic TA dinucleotide sites. I, intron; p(A), polyadenylation sequence.
Figure 6. Targeting of cis CAGGS-BΔcFVIII _SB_-Tns to LSECs and correction of the bleeding diathesis in knockout hemophilia A mice.
(A) Mice treated with 25 μg of cis _SB_-Tn in HA s50 nanocapsules were bled 5, 12, 16, 19, and 50 weeks after injection and aPTTs determined in duplicate as described in Methods. Treated mice (n = 6) had aPTTs of 25.5 ± 3.1 seconds at 5 weeks and 28.8 ± 3.7 seconds at 50 weeks; these were not significantly different from those of age-matched wild-type (n = 3) aPTTs of 23.5 ± 1.3 seconds (5 weeks; light gray) and 27.9 ± 1.6 seconds (50 weeks, dark gray). In contrast, untreated mice (n = 3) had aPTTs ranging from 46.7 ± 3.5 to 65.7 ± 9.6 seconds. The data show the mean ± 1 SD. *P < 0.001 compared with untreated hemophilia A mice. Far-right dark gray bar, plasma from a separate group of untreated hemophilia A mice collected and assayed with the other 50-week samples. (B) Coamatic determination of FVIII activity in mice. FVIII activity in plasma samples was also determined using the Coamatic assay, which measures the conversion of FX to FXa mediated by FIXa and its cofactor FVIII. The graph shows the mean ± 1 SD of values obtained using the procedure outlined in Methods and wild-type mouse plasma as positive control. By 5 weeks after injection, the treated animals showed greater than 95% wild-type mouse plasma activity; and this increased to greater than 100% by 50 weeks. In contrast, the plasma from untreated hemophilia A mice exhibited no detectable activity. ANOVA analysis indicated no significant difference between the plasma from treated hemophilia A mice and that from wild-type animals.
Figure 7. PCR identification of DNA flanking the _SB_-Tn insertion sites.
(A) DNA isolated from the livers of hemophilia A mice injected with HA pT2/CAGGS-BΔcFVIII//IF_SB_10 nanocapsules and wild-type controls served as template using primer pairs specific for the FVIII hemophilia A knockout (top) or the wild-type allele (bottom). (B) Lack of _SB_10 CDS persistence in the HA-treated knockout mice. PCR amplification of the _SB_10 CDS was performed (19) using liver DNA isolated from the DsRed2 animals and treated hemophilia A mice. The size or identity of the predicted amplicons and selected bands of the DNA marker (M) are indicated. The time (after injection) that the livers were harvested is indicated above the gels. (C) Schematic of the inverted-nested PCR strategy used to identify DNA flanking the _SB_-Tn insertion site. The genomic DNA digested by NcoI or XhoI was subjected to self-ligation and the products used as template for the initial inverted PCR amplification with primer pairs RP1/LP1 and RNP1/FNP1 for XhoI- and NcoI-digested DNA, respectively. The second PCR amplification used the XhoI and NcoI initial reactions as template with internal nested primer pairs LP2/RP2 and RNP2/FNP2, respectively. The PCR products were analyzed by agarose gel electrophoresis and visualized by UV light after ethidium bromide staining. The size of the 3 heavy bands is shown to the left of the NcoI gel. (D) Identification of the insertion sites in hemophilia A mice treated with HA-encapsulated pT2/CAGGS-BΔcFVIII//IF_SB_10. The region of the ID/DR of the Tn and the requisite duplicated TA (gray) followed by 40 nt of genomic DNA flanking the identified insertion sites are indicated above the sequences. The chromosomal location established by BLAST analysis is shown at right, and the intronic insertion sites are marked with asterisks. The closest adjacent genes for the other insertions are listed, with the distance in kb from the Tn insertion indicated in parentheses if less than 100 kb.
Similar articles
- Phenotypic correction and long-term expression of factor VIII in hemophilic mice by immunotolerization and nonviral gene transfer using the Sleeping Beauty transposon system.
Ohlfest JR, Frandsen JL, Fritz S, Lobitz PD, Perkinson SG, Clark KJ, Nelsestuen G, Key NS, McIvor RS, Hackett PB, Largaespada DA. Ohlfest JR, et al. Blood. 2005 Apr 1;105(7):2691-8. doi: 10.1182/blood-2004-09-3496. Epub 2004 Dec 2. Blood. 2005. PMID: 15576475 - Sustained FVIII expression and phenotypic correction of hemophilia A in neonatal mice using an endothelial-targeted sleeping beauty transposon.
Liu L, Mah C, Fletcher BS. Liu L, et al. Mol Ther. 2006 May;13(5):1006-15. doi: 10.1016/j.ymthe.2005.11.021. Epub 2006 Feb 7. Mol Ther. 2006. PMID: 16464640 - Factor VIII can be synthesized in hemophilia A mice liver by bone marrow progenitor cell-derived hepatocytes and sinusoidal endothelial cells.
Yadav N, Kanjirakkuzhiyil S, Ramakrishnan M, Das TK, Mukhopadhyay A. Yadav N, et al. Stem Cells Dev. 2012 Jan;21(1):110-20. doi: 10.1089/scd.2010.0569. Epub 2011 Jun 1. Stem Cells Dev. 2012. PMID: 21480781 - Development of improved factor VIII molecules and new gene transfer approaches for hemophilia A.
Saenko EL, Ananyeva NM, Moayeri M, Ramezani A, Hawley RG. Saenko EL, et al. Curr Gene Ther. 2003 Feb;3(1):27-41. doi: 10.2174/1566523033347417. Curr Gene Ther. 2003. PMID: 12553533 Review. - Clinical gene transfer studies for hemophilia A.
Chuah MK, Collen D, VandenDriessche T. Chuah MK, et al. Semin Thromb Hemost. 2004 Apr;30(2):249-56. doi: 10.1055/s-2004-825638. Semin Thromb Hemost. 2004. PMID: 15118936 Review.
Cited by
- Ultrasound-mediated gene delivery specifically targets liver sinusoidal endothelial cells for sustained FVIII expression in hemophilia A mice.
Lawton SM, Manson MA, Fan MN, Chao TY, Chen CY, Kim P, Campbell C, Cai X, Vander Kooi A, Miao CH. Lawton SM, et al. Mol Ther. 2024 Apr 3;32(4):969-981. doi: 10.1016/j.ymthe.2024.02.010. Epub 2024 Feb 9. Mol Ther. 2024. PMID: 38341614 - Liver-Targeting Nanoplatforms for the Induction of Immune Tolerance.
Kusumoputro S, Au C, Lam KH, Park N, Hyun A, Kusumoputro E, Wang X, Xia T. Kusumoputro S, et al. Nanomaterials (Basel). 2023 Dec 26;14(1):67. doi: 10.3390/nano14010067. Nanomaterials (Basel). 2023. PMID: 38202522 Free PMC article. Review. - Current, emerging, and potential therapies for non-alcoholic steatohepatitis.
Yang Z, Wang L. Yang Z, et al. Front Pharmacol. 2023 Mar 30;14:1152042. doi: 10.3389/fphar.2023.1152042. eCollection 2023. Front Pharmacol. 2023. PMID: 37063264 Free PMC article. Review. - Three-dimensional (3D) liver cell models - a tool for bridging the gap between animal studies and clinical trials when screening liver accumulation and toxicity of nanobiomaterials.
Tutty MA, Movia D, Prina-Mello A. Tutty MA, et al. Drug Deliv Transl Res. 2022 Sep;12(9):2048-2074. doi: 10.1007/s13346-022-01147-0. Epub 2022 May 4. Drug Deliv Transl Res. 2022. PMID: 35507131 Free PMC article. Review. - Defenestrated endothelium delays liver-directed gene transfer in hemophilia A mice.
Kaminski TW, Ju EM, Gudapati S, Vats R, Arshad S, Dubey RK, Katoch O, Tutuncuoglu E, Frank J, Brzoska T, Stolz DB, Watkins SC, Chan SY, Ragni MV, Novelli EM, Sundd P, Pradhan-Sundd T. Kaminski TW, et al. Blood Adv. 2022 Jun 28;6(12):3729-3734. doi: 10.1182/bloodadvances.2021006388. Blood Adv. 2022. PMID: 35427414 Free PMC article.
References
- Ponder K.P. Gene therapy for hemophilia. Curr. Opin. Hematol. 2006;13:301–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK067436/DK/NIDDK NIH HHS/United States
- R01-HL081582-02/HL/NHLBI NIH HHS/United States
- HL0258591-01/HL/NHLBI NIH HHS/United States
- R01DK067436-01/DK/NIDDK NIH HHS/United States
- R01 HL081582/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical