Signalling through C-type lectin receptors: shaping immune responses - PubMed (original) (raw)
Review
Signalling through C-type lectin receptors: shaping immune responses
Teunis B H Geijtenbeek et al. Nat Rev Immunol. 2009 Jul.
Abstract
C-type lectin receptors (CLRs) expressed by dendritic cells are crucial for tailoring immune responses to pathogens. Following pathogen binding, CLRs trigger distinct signalling pathways that induce the expression of specific cytokines which determine T cell polarization fates. Some CLRs can induce signalling pathways that directly activate nuclear factor-kappaB, whereas other CLRs affect signalling by Toll-like receptors. Dissecting these signalling pathways and their effects on host immune cells is essential to understand the molecular mechanisms involved in the induction of adaptive immune responses. In this Review we describe the role of CLR signalling in regulating adaptive immunity and immunopathogenesis and discuss how this knowledge can be harnessed for the development of innovative vaccination approaches.
Figures
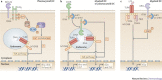
Figure 1. DC-SIGN signalling modulates TLR signalling through RAF1-dependent acetylation of p65.
a | DC-specific ICAM3-grabbing non-integrin (DC-SIGN) binding to pathogens such as HIV-1, Mycobacterium tuberculosis and Candida albicans activates the small GTPase Ras proteins, which associate with the serine/threonine protein kinase RAF1 to allow its phosphorylation at residues Ser338, and Tyr340 and Tyr341 by p21-activated kinases (PAKs) and Src kinases, respectively. The upstream effectors that activate PAKs and Src kinases are unknown but might involve leukaemia-associated Rho guanine nucleotide exchange factor (LARG) and Ras homologue A (RHOA), as these proteins are activated by HIV-1 binding to DC-SIGN. RAF1 activation leads to modulation of Toll-like receptor (TLR)-induced nuclear factor-κB (NF-κB) activation. TLR3 and TLR4 activation by double-stranded RNA (dsRNA) and lipopolysaccharide (LPS), respectively, leads to the recruitment of the ubiquitin ligase TNF receptor-associated factor 6 (TRAF6) by myeloid differentiation primary response protein 88 (MYD88), TIR-domain-containing adaptor protein inducing IFNβ (TRIF) and other factors (not depicted). Self-induced polyubiquitylation (Ub) of TRAF6 mediates the recruitment of TGFβ-activating kinase 1 (TAK1), which activates the IκB kinase (IKK) complex. IKKβ phosphorylates inhibitor of NF-κBα (IκBα), thereby targeting it for proteasomal degradation and releasing NF-κB from inhibition, which then translocates into the nucleus, where it binds to NF-κB sites in the enhancer and promoter regions of target genes. RAF1 induces the phosphorylation of p65 at Ser276 through an unknown pathway. Phosphorylated Ser276 serves as a binding site for the histone acetyltransferases CREB-binding protein (CBP) and p300 (not depicted) to acetylate (Ac) p65 at different lysines. Acetylated p65 exhibits enhanced transcriptional activity as well as prolonged nuclear presence owing to impaired binding by IκB, which increases the rate of Il10 (interleukin-10) transcription and prolongs the binding of NF-κB to the Il10 promoter, thereby increasing the production of IL-10. b | Binding of the salivary protein Salp15 from the tick Ixodes scapularis to DC-SIGN activates RAF1, but co-ligation with another receptor, presumably CD4, changes downstream effectors of RAF1, leading to MEK (MAPK/ERK kinase) but not ERK (extracellular signal-regulated kinase) activation. The spirochete Borrelia burgdorferi exploits the effects of Salp15 to infect humans. Salp15-induced MEK-dependent signalling decreases _B. burgdorferi_-induced TLR1–TLR2-dependent pro-inflammatory cytokine production by enhancing the decay of Il6 and Tnf (tumour necrosis factor) mRNA while impairing nucleosome remodelling at the Il12a promoter, which is required for transcriptional initiation. MAL, MYD88-adaptor-like protein.
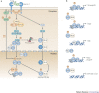
Figure 2. Signalling by BDCA2, DCIR and MICL antagonizes TLR signalling.
a | Activation of blood DC antigen 2 protein (BDCA2) leads to the recruitment of spleen tyrosine kinase (SYK) to the phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) of the paired signalling adaptor Fc receptor γ-chain (FcRγ). SYK activation leads to the activation of a complex consisting of B cell linker (BLNK), Bruton's tyrosine kinase (BTK) and phospholipase Cγ2 (PLCγ2), which induces Ca2+ mobilization. The signalling pathway downstream of this complex is not fully known, but results in the downregulation of Toll-like receptor 9 (TLR9)-induced production of interferon-α (IFNα), IFNβ, tumour necrosis factor (TNF) and interleukin-6 (IL-6) by plasmacytoid dendritic cells (DCs). Calcium mobilization might be involved in inhibiting the recruitment of myeloid differentiation primary response protein 88 (MYD88) and thereby reducing the production of TLR-induced cytokines. b | Activation of DC immunoreceptor (DCIR) leads to its internalization into endosomal compartments, where TLR8 and TLR9 reside. The phosphorylation of its immunoreceptor tyrosine-based inhibitory motif (ITIM) recruits the phosphatases SH2-domain-containing protein tyrosine phosphatase 1 (SHP1) or SHP2, which induces the activation of an unidentified signalling pathway that leads to the downregulation of TLR8-induced IL-12 and TNF production or TLR9-induced IFNα and TNF production by either myeloid or plasmacytoid DCs, respectively. c | Cross-linking of myeloid C-type lectin-like receptor (MICL) on myeloid DCs also results in the phosphorylation of its ITIM and the recruitment of SHP1 or SHP2. MICL activation has been shown to induce the activation of extracellular signal-regulated kinase (ERK). However, it is not known whether ERK activation is involved in the downregulation of TLR4-induced IL-12 production. LPS, lipopolysaccharide; MAL, MYD88-adaptor-like protein; TRAM, TRIF-related adaptor molecule; TRIF, TIR-domain-containing adaptor protein inducing IFNβ.
Figure 3. Dectin 1 signalling through SYK and RAF1 directs NF-κB-mediated cytokine expression.
a | The binding of fungi to DC-associated C-type lectin 1 (dectin 1) induces phosphorylation of the YxxL (in which x denotes any amino acid) motif in its cytoplasmic domain. Spleen tyrosine kinase (SYK) is recruited to the two phosphorylated receptors, which leads to the formation of a complex involving CARD9 (caspase recruitment domain family, member 9), B cell lymphoma 10 (BCL-10) and mucosa-associated lymphoid tissue lymphoma translocation gene 1 (MALT1); this induces the activation of the IκB kinase (IKK) complex through an unknown pathway. IKKβ phosphorylates inhibitor of NF-κBα (IκBα), thereby targeting it for proteasomal degradation. This results in the release of nuclear factor-κB (NF-κB; consisting of either p65–p50 or REL–p50 dimers), which then translocates into the nucleus. SYK activation also leads to the activation of the non-canonical NF-κB pathway that is mediated by NF-κB inducing kinase (NIK) and IKKα, which target p100 for proteolytic processing to p52; this subsequently leads to nuclear translocation of RELB–p52 dimers. In a SYK-independent manner, dectin 1 activation leads to the phosphorylation and activation of the serine/threonine protein kinase RAF1 by Ras proteins, which leads to the phosphorylation of p65 at Ser276. Phosphorylated Ser276 serves as a binding site for the histone acetyltransferases CREB-binding protein (CBP) or p300 (not depicted) to acetylate (Ac) p65 at different lysine residues. Ser276-phosphorylated p65 also dimerizes with RELB to form inactive dimers that cannot bind DNA, and hence attenuates the transcriptional activity of RELB. b | Binding of acetylated p65 to the Il10 (interleukin-10) enhancer and Il6 promoter increases the transcription of both genes. The RAF1-mediated formation of inactive p65–RELB dimers results in the binding of acetylated p65 to the Il12b promoter and REL–p50 to the Il1b promoter, which leads to increased IL-12p40 and IL-1β expression, respectively. The higher DNA binding affinity of acetylated p65 displaces REL–p50 dimers from the Il12a and Il23p19 promoters, which leads to increased expression of IL-12p35. However, the expression of IL-23p19 is decreased, as REL–p50 dimers are stronger transactivators of Il23p19 than p65–p50 dimers. The formation of inactive p65–RELB dimers blocks binding of RELB–p52 to the promoters of the chemokine genes Ccl17 (CC-chemokine ligand 17) and Ccl22, thereby blocking chemokine expression.
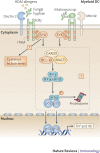
Figure 4. Signalling by dectin 2 and mincle leads to cytokine expression.
Both DC-associated C-type lectin 2 (dectin 2) and macrophage-inducible C-type lectin (mincle) pair with the signalling adaptor molecule Fc receptor γ-chain (FcRγ) through the presence of a positively charged amino acid residue in their transmembrane regions. The phosphorylation of the immunoreceptor tyrosine-based activation motifs (ITAMs) of FcRγ following C-type lectin receptor (CLR) activation serves to recruit spleen tyrosine kinase (SYK) and induces signalling pathways that modulate cytokine expression. Dectin 2 binds to pathogen-associated molecular patterns (PAMPs) expressed by fungal hyphae, and mincle binds to α-mannosyl PAMPs on Malassezia spp. fungi. Both signalling pathways lead to Toll-like receptor (TLR)-independent production of cytokines such as tumour necrosis factor (TNF) and interleukin-6 (IL-6); dectin 2 triggering is known to result in nuclear factor-κB (NF-κB) p50–p65 activation, and mincle triggering induces a CARD9 (caspase recruitment domain family, member 9)-dependent signalling pathway. Similarities with the dectin 1 signalling pathway suggest that both these CLRs couple SYK activation to NF-κB activation using a complex involving CARD9, B-cell lymphoma-10 (BCL-10) and mucosa-associated lymphoid tissue lymphoma translocation gene 1 (MALT1). Dectin 2 binding of house dust mite (HDM) allergens activates SYK through FcRγ to generate cysteinyl leukotrienes, which are secreted and mediate allergic inflammation in lungs.
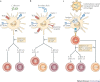
Figure 5. CLR signalling can be harnessed in vaccination approaches to tailor adaptive immune responses to pathogens.
a | Simultaneous triggering of DC-specific ICAM3-grabbing non-integrin (DC-SIGN) and DC-associated C-type lectin 1 (dectin 1) on dendritic cells (DCs) by Candida albicans induces a T helper 1 (TH1) and TH17 cell-mediated immune response that is essential to clear the fungi. Binding of fungi to dectin 1 triggers the spleen tyrosine kinase (SYK)-dependent activation of nuclear factor-κB (NF-κB) dimers p65–p50, REL–p50 and RELB–p52 (see Fig. 2). Both DC-SIGN and dectin 1 induce the RAF1 pathway, which controls NF-κB activation by inducing p65 acetylation and attenuating the activity of the RELB–p52 dimer. The sequestration of RELB into an inactive RELB–p65 dimer allows the induction of interleukin-1β (IL-1β), IL-12p70 and IL-23 production and therefore the induction of TH1 and TH17 cells. Inhibition of RAF1 abrogates the expression of cytokines that promote TH1 and TH17 cell development and induces TH2 cell responses. b | Mycobacterium tuberculosis interacts with TLRs, DC-SIGN, mannose receptor and dectin 1 and induces a TH1 and TH17 cell response. Although the signalling pathways that are activated by M. tuberculosis have not been elucidated completely, M. tuberculosis has been shown to trigger DC-SIGN to induce the RAF1 pathway and dectin 1 to induce SYK-dependent NF-κB activation. Integration of these pathways results in a TH1 and TH17 cell response. Inhibition of RAF1 might induce a TH2 cell response given that dectin 1-induced SYK signalling in the absence of RAF1 activation inhibits the production of cytokines that promote TH1 and TH17 cell development. c | Vaccination strategies that specifically target DCs can harness the antigen presentation as well as the signalling ability of CLRs to induce strong tailored immune responses to specific antigens. A carbohydrate-coated microparticle containing antigens can specifically target dectin 1 and DC-SIGN, leading to efficient uptake and presentation of the antigen. Triggering of both dectin 1 and DC-SIGN by the carbohydrate ligands on the microparticle could induce specific TH1 and TH17 cell responses. Differential activation of RAF1 or SYK allows the modulation of the immune responses.
Similar articles
- Targeting Syk-Card9-activating C-type lectin receptors by vaccine adjuvants: findings, implications and open questions.
Lang R, Schoenen H, Desel C. Lang R, et al. Immunobiology. 2011 Nov;216(11):1184-91. doi: 10.1016/j.imbio.2011.06.005. Epub 2011 Jun 21. Immunobiology. 2011. PMID: 21742403 Review. - Innate signaling and regulation of Dendritic cell immunity.
van Vliet SJ, den Dunnen J, Gringhuis SI, Geijtenbeek TB, van Kooyk Y. van Vliet SJ, et al. Curr Opin Immunol. 2007 Aug;19(4):435-40. doi: 10.1016/j.coi.2007.05.006. Epub 2007 Jul 12. Curr Opin Immunol. 2007. PMID: 17629469 Review. - Endogenous ligands for C-type lectin receptors: the true regulators of immune homeostasis.
García-Vallejo JJ, van Kooyk Y. García-Vallejo JJ, et al. Immunol Rev. 2009 Jul;230(1):22-37. doi: 10.1111/j.1600-065X.2009.00786.x. Immunol Rev. 2009. PMID: 19594627 Review. - The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells.
Schreibelt G, Klinkenberg LJ, Cruz LJ, Tacken PJ, Tel J, Kreutz M, Adema GJ, Brown GD, Figdor CG, de Vries IJ. Schreibelt G, et al. Blood. 2012 Mar 8;119(10):2284-92. doi: 10.1182/blood-2011-08-373944. Epub 2012 Jan 10. Blood. 2012. PMID: 22234694 - Dendritic cell-associated lectin 2 (DCAL2) defines a distinct CD8α- dendritic cell subset.
Kasahara S, Clark EA. Kasahara S, et al. J Leukoc Biol. 2012 Mar;91(3):437-48. doi: 10.1189/jlb.0711384. Epub 2011 Dec 6. J Leukoc Biol. 2012. PMID: 22147811 Free PMC article.
Cited by
- Organization of the extracellular portion of the macrophage galactose receptor: a trimeric cluster of simple binding sites for N-acetylgalactosamine.
Jégouzo SA, Quintero-Martínez A, Ouyang X, dos Santos Á, Taylor ME, Drickamer K. Jégouzo SA, et al. Glycobiology. 2013 Jul;23(7):853-64. doi: 10.1093/glycob/cwt022. Epub 2013 Mar 18. Glycobiology. 2013. PMID: 23507965 Free PMC article. - Parasitic infections: a role for C-type lectins receptors.
Vázquez-Mendoza A, Carrero JC, Rodriguez-Sosa M. Vázquez-Mendoza A, et al. Biomed Res Int. 2013;2013:456352. doi: 10.1155/2013/456352. Epub 2013 Jan 27. Biomed Res Int. 2013. PMID: 23509724 Free PMC article. Review. - Dendritic cell expression of the C-type lectin receptor CD209a: A novel innate parasite-sensing mechanism inducing Th17 cells that drive severe immunopathology in murine schistosome infection.
Ponichtera HE, Stadecker MJ. Ponichtera HE, et al. Exp Parasitol. 2015 Nov;158:42-7. doi: 10.1016/j.exppara.2015.04.006. Epub 2015 Apr 23. Exp Parasitol. 2015. PMID: 25913088 Free PMC article. - HUMAN MICROBIOTA. Small molecules from the human microbiota.
Donia MS, Fischbach MA. Donia MS, et al. Science. 2015 Jul 24;349(6246):1254766. doi: 10.1126/science.1254766. Epub 2015 Jul 23. Science. 2015. PMID: 26206939 Free PMC article. Review. - Glycosylation in SARS-CoV-2 variants: A path to infection and recovery.
Aloor A, Aradhya R, Venugopal P, Gopalakrishnan Nair B, Suravajhala R. Aloor A, et al. Biochem Pharmacol. 2022 Dec;206:115335. doi: 10.1016/j.bcp.2022.115335. Epub 2022 Oct 31. Biochem Pharmacol. 2022. PMID: 36328134 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources