The Arp2/3 complex and WASp are required for apical trafficking of Delta into microvilli during cell fate specification of sensory organ precursors - PubMed (original) (raw)
The Arp2/3 complex and WASp are required for apical trafficking of Delta into microvilli during cell fate specification of sensory organ precursors
Akhila Rajan et al. Nat Cell Biol. 2009 Jul.
Abstract
Cell fate decisions mediated by the Notch signalling pathway require direct cell-cell contact between adjacent cells. In Drosophila melanogaster, an external sensory organ (ESO) develops from a single sensory organ precursor (SOP) and its fate specification is governed by differential Notch activation. Here we show that mutations in actin-related protein-3 (Arp3) compromise Notch signalling, leading to a fate transformation of the ESO. Our data reveal that during ESO fate specification, most endocytosed vesicles containing the ligand Delta traffic to a prominent apical actin-rich structure (ARS) formed in the SOP daughter cells. Using immunohistochemistry and transmission electron microscopy (TEM) analyses, we show that the ARS contains numerous microvilli on the apical surface of SOP progeny. In Arp2/3 and WASp mutants, the surface area of the ARS is substantially reduced and there are significantly fewer microvilli. More importantly, trafficking of Delta-positive vesicles from the basal area to the apical portion of the ARS is severely compromised. Our data indicate that WASp-dependent Arp2/3 actin polymerization is crucial for apical presentation of Delta, providing a mechanistic link between actin polymerization and Notch signalling.
Figures
Figure 1
Arp3 mutations cause a pIIa-to-pIIb transformation in the ESO lineage (a) A diagram of the ESO lineage in wild-type (WT) and in Notch loss-of-function background. Each cell is represented by a circle; the cells in which Notch is activated are in purple and the signal-sending cells are in green. The dashed lines indicate daughter cells in which Notch is activated. (b) Homozygous clones of Arp383F on an adult thorax induced by Ubx–FLP. The clone (dashed lines) is identified by an epithelial cell marker multiple wing hair (mwh), which marks the trichomes (small hairlike structures) on epithelial cells. Mutant clones show loss of external structures, socket and shaft cells, of the microchaetae. Macrochaetae (arrow) sometimes show a double-shaft phenotype in Arp383F clones. (b´) Higher magnification of an Arp383F clone shows that rarely there are shaft and sockets (arrows) in the mutant clone. Most of the Arp383F clones show a balding phenotype. (c) Schematic representation of the mapping strategy. The inverted triangles represent P elements that were used for recombination mapping. Deficiencies represented by lines: those in red failed to complement the alleles, whereas those in green complement our alleles. (d) Rescue of the Arp3 phenotype by overexpression of an Arp3 cDNA construct in the mutant clones. An image of a pupal notum of an adult thorax which harbours Arp3 clones; the mutant clones (dashed lines) do not show bristle loss (compare with b). (e) Flies that harbour clones of Arpc1Q25st show bald patches. The clones were not generated in a Minute background and hence appear smaller than Arp3 clones (compare with b). We have not outlined the clones in Arpc1Q25st as they are unmarked clones. (f, g) A projection of confocal slices show part of the notum at 24–26 h APF stained for ELAV (red) and Cut (green). All cells in the wild-type (f) sensory clusters are positive for Cut and one of the cells is ELAV-positive. In Arp3 (g) mutant clones all of the cells in the sensory clusters are positive for Cut and ELAV, indicating the transformation of all SOP progeny to neurons. Scale bar, 10 µm.
Figure 2
Arp3 is required in the signal sending cells during Notch signalling (a) Overexpression of NECN in wild-type SOPs using the sca109-68-GAL4 driver results in a multiple socket phenotype in the majority of the sensory clusters. We generated Arp3 clones (dashed line) using Ubx–FLP in this NECN overexpression background. We did not observe a region of bald cuticle in the Arp3 clones. (b) Clones of Arp3515FC induced by hs-FLP in follicle cells are marked by the absence of GFP (green). FasciclinIII (red) marks the follicle cells and is upregulated in polar follicle cells. Phalloidin (blue) marks the membrane of all cells. When polar follicle cells are wild-type (WT), stalk cells (yellow arrow) are formed normally, separating two cysts, whereas, when the polar follicle cells are mutant for Arp3, we found a loss of stalk cells between the cysts, resulting in a partial fusion of cysts (white arrow). (c–c´´) The follicle cells of the cyst harbour mutant clones of Arp3 induced by hs-FLP at stage 7 of oogenesis. Arp3 mutant clones are marked by the absence of nuclear GFP (green). The cyst was immunostained for Hnt (red), a Notch downstream target gene in the follicle cells. Note that Hnt is still expressed in the Arp3 mutant follicle cell clones (non-green cells). (d) Overexpression of Delta in WT cells (green) near the dorsal-ventral boundary of the wing can induce Cut expression (red) in the adjacent cells near the dorsal-ventral boundary at the dorsal compartment. (e) Overexpression of Delta (blue) in Arpc1 mutant cells (green) cannot induce Cut expression (red) in the adjacent cells near the dorsal-ventral boundary at the dorsal compartment. Note the loss of Cut expression when the clone crosses the dorsal-ventral boundary (arrow). Scale bars, 10 µm (b, d) and 5 µm (c).
Figure 3
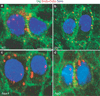
Delta is normally endocytosed in Arp3 and Arpc1 mutant pIIa-pIIb. (a–d) Endocytosis assay for Delta ligand (red) performed at the 2-cell stage in pIIa-pIIb. Sens (blue) labels the nucleus and Dlg (green) marks the sub-apical membrane. A projection of optical slices shows that in the negative control (shits1 (b), Delta (red) is found only on the membrane and not in cytoplasmic vesicles between the nucleus and membrane. However, in the wild-type (WT, a), Arpc1 (c) and Arp3 (d) pIIa-pIIb, endocytosed Delta vesicles (red) are present in the cytoplasm, indicating that Arp2/3 function is not required for Delta endocytosis. Note small punctae in b when Delta is not endocytosed. Scale bar, 5 µm.
Figure 4
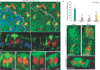
The ARS forms specifically in the pIIa-pIIb progeny and is reduced in Arp3, Arpc1 and WASp mutant SOP progeny. (a, a´) A projection of confocal sections shows that the ARS identified by phalloidin (green) staining is present in both wild-type (WT, white arrow) pIIa-pIIb and Arpc1 (yellow arrow) mutant pIIa-pIIb cells marked by Sens (red). Arpc1 homozygous mutant clones (dotted lines) are marked by the absence of nuclear GFP (blue). (a´´) An orthogonal confocal section shows that the ARS is quite broad in the WT pIIa-pIIb (white arrow) and has an umbrella-shaped structure, whereas the ARS in the Arpc1 homozygous clones (yellow arrow) seems compressed and the lateral ‘stalk’ of the ARS is malformed. (b) Quantification of the apical area of the ARS in different genotypes. The ARS area was quantified using the Measure function of ImageJ software. The measurements were analysed using a Student’s _t_-test (***P <0.0001). Data are mean ± s.e.m. and the number of SOP progeny pairs used for quantification per genotype is indicated in the bars. (c–g´´) Pupal nota stained with Sens (red) and phalloidin (green) reveal ARS in pIIa-pIIb. Projections of orthogonal slices show the ARS in WT (c, white arrow), Arpc1 (d, yellow arrow), α-adaptin (e), numb (f) and neuralized (g–g´´) pIIa-pIIb. An apical section (g) reveals apical (0.5 µm) actin enrichment whereas a basal section (g´) of the sample (~6 µm) shows the nuclei of the SOP progeny. Scale bars, 10 µm (a, a´´, g, g´´) and 5 µm (c–f).
Figure 5
TEM analysis reveals enrichment of actin-filled finger-like projections in pIIa-pIIb cells at 18 h APF. (a, d) Orthogonal sections of wild-type (WT, a) and Arp3 (d) pIIa-pIIb cells show finger-like projections (arrows) at the apical domain of the cells. (b–f) Cross-section of WT (b) and Arp3 (e) pIIa-pIIb cells show finger-like projections (arrows). (c, f) Higher magnification of the apical surface of WT (c) and Arp3 (f) pIIa-pIIb cells shows actin bundles (arrows) inside the finger-like projections. (g) Quantification of the number of finger-like projections at the 2-cell stage in WT and Arp3. The total number of microvilli in SOP and epithelial cells were quantified using ImageJ. The data are mean ± s.e.m and measurements were analysed using Student’s _t_-test. Three SOP progeny pairs were used for this quantification per genotype. (h) Schematic representation of pIIa-pIIb in the prepupal thorax epithelium. The asterisk represents the level of the first electron microscopy section at 60 nm. Abbreviations: cuticle (Cu), chitin fibre (CF), epithelial cell (EC), sensory organ precursor cell (SOP). Scale bars, 0.5 µm (a, b, d, e) and 0.1 µm (c, f).
Figure 6
Finger-like projections in pIIa-pIIb cells are enriched with F-actin bundles and are marked by a microvillar marker Myo1B. (a, a´) Immuno-electron microscopy image of an orthogonal section through the wild-type pIIa-pIIb of a pupal notum shows an enrichment of phalloidin (electron-dense material) in the finger-like projections along the apical region of the ARS. (a´) A higher magnification view of the boxed region in a is shown in a´. The arrow points to the enrichment of phalloidin in the finger-like projections (b–b´´´) Confocal images of single optical (xy axis) sections (b–b´´) and orthogonal section (b´´´) of wild-type (WT) pIIa-pIIb cells immunostained for Myo1B (red), phalloidin (green) and Sens (blue). Scale bars, 0.5 µm (a, a´) and 5 µm (b, b´´´).
Figure 7
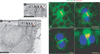
Delta localization to the ARS is reduced in Arpc1 mutants. (a–c´´) Pupal wing nota at the 2-cell SOP stage (18.30 h APF) were immunostained with phalloidin (green) and Delta (magenta). Arpc1 homozygous mutant cells are marked by the absence of GFP (blue). (a, b) A single section along the xy axis through pIIa-pIIb cells shows an enrichment of Delta on the ARS in wild-type (WT, a) and this enrichment is much reduced in Arpc1 (b). (a´, b´) A single section along the xy axis of pIIa-pIIb shows that the Delta vesicles colocalize along the lateral stalk of the ARS in WT and in the basal portion of the umbrella region of the ARS (a´). In Arpc1 (b´), the lateral stalk of the ARS is malformed and there is a reduction in the number of Delta vesicles that colocalize on the apical portion of the ARS. (c–c´´) A projection of confocal sections of a pupal notum harbouring an Arpc1 mutant clone (dashed line). In the WT region, a high density of Delta vesicles are clustered on and around the ARS, whereas in the mutant clones, the Delta vesicles are more widely distributed and do not cluster around the ARS; compare arrowheads (Arpc1) with arrows (WT). Scale bars, 5 µm (a, a´) and 10 µm (c).
Figure 8
Arp2/3 and WASp are required for trafficking of endocytosed Delta to the apical ARS 1 h post-endocytosis. (a–f´´) A pulse-chase assay for the trafficking of endocytosed Delta (magenta) at different time-points with respect to the ARS (green) was performed in live pupal nota of wild-type (WT) and Arp3 mutants. Confocal images show apical (0.5 µm), basal (6 µm) and orthogonal sections of the pIIa-pIIb cells of the WT notum (a–a´´, c–c´´, e–e´´), and Arp3 mutant clones (b–b´´, d–d´´, f–f´´). The pulse-chase assays for three different time-points, 0 min, 30 min and 60 min, are shown. (g) Quantification of the number of internalized Delta vesicles that are present apically and colocalize with the ARS. Measurements of total number of Delta vesicles that traffic to the ARS 1 h after chase were analysed using a Student’s _t_-test (***P < 0.0001). Data are mean ± s.e.m. and the number of SOP progeny pairs quantified per genotype is indicated in the bars. Note that fewer vesicles that colocalize in mutants when compared with the WT control and the difference is statistically significant. (h) Proposed model. In the pIIb cell, Delta is endocytosed by Neuralized (Neur) and trafficked by Epsin to an endocytic compartment where it undergoes activation, probably by a proteolytic cleavage event. It is trafficked back to the membrane in a compartment positive for Rab11 (ref. 12) and the exocyst complex member Sec15 (ref. 13). Arp2/3 and WASp are required for the formation of branched actin networks to form the ‘stalk’ of the ARS and enables endocytosed vesicles containing activated Delta to traffic back to the dense actin-rich microvilli at the apical membrane of the pIIb cell, where it can signal. Scale bars, 5 µm.
Comment in
- Delta traffic takes a sh-Arp turn.
Schejter ED. Schejter ED. Nat Cell Biol. 2009 Jul;11(7):791-3. doi: 10.1038/ncb0709-791. Nat Cell Biol. 2009. PMID: 19568264
Similar articles
- Activation of Arp2/3 by WASp Is Essential for the Endocytosis of Delta Only during Cytokinesis in Drosophila.
Trylinski M, Schweisguth F. Trylinski M, et al. Cell Rep. 2019 Jul 2;28(1):1-10.e3. doi: 10.1016/j.celrep.2019.06.012. Cell Rep. 2019. PMID: 31269431 - AP-1 controls the trafficking of Notch and Sanpodo toward E-cadherin junctions in sensory organ precursors.
Benhra N, Lallet S, Cotton M, Le Bras S, Dussert A, Le Borgne R. Benhra N, et al. Curr Biol. 2011 Jan 11;21(1):87-95. doi: 10.1016/j.cub.2010.12.010. Epub 2010 Dec 30. Curr Biol. 2011. PMID: 21194948 - Par3 cooperates with Sanpodo for the assembly of Notch clusters following asymmetric division of Drosophila sensory organ precursor cells.
Houssin E, Pinot M, Bellec K, Le Borgne R. Houssin E, et al. Elife. 2021 Oct 1;10:e66659. doi: 10.7554/eLife.66659. Elife. 2021. PMID: 34596529 Free PMC article. - WASP family proteins, more than Arp2/3 activators.
Tyler JJ, Allwood EG, Ayscough KR. Tyler JJ, et al. Biochem Soc Trans. 2016 Oct 15;44(5):1339-1345. doi: 10.1042/BST20160176. Biochem Soc Trans. 2016. PMID: 27911716 Free PMC article. Review. - New insights into the regulation and cellular functions of the ARP2/3 complex.
Rotty JD, Wu C, Bear JE. Rotty JD, et al. Nat Rev Mol Cell Biol. 2013 Jan;14(1):7-12. doi: 10.1038/nrm3492. Epub 2012 Dec 5. Nat Rev Mol Cell Biol. 2013. PMID: 23212475 Review.
Cited by
- Optical tweezers studies on Notch: single-molecule interaction strength is independent of ligand endocytosis.
Shergill B, Meloty-Kapella L, Musse AA, Weinmaster G, Botvinick E. Shergill B, et al. Dev Cell. 2012 Jun 12;22(6):1313-20. doi: 10.1016/j.devcel.2012.04.007. Epub 2012 May 31. Dev Cell. 2012. PMID: 22658935 Free PMC article. - dEHBP1 controls exocytosis and recycling of Delta during asymmetric divisions.
Giagtzoglou N, Yamamoto S, Zitserman D, Graves HK, Schulze KL, Wang H, Klein H, Roegiers F, Bellen HJ. Giagtzoglou N, et al. J Cell Biol. 2012 Jan 9;196(1):65-83. doi: 10.1083/jcb.201106088. Epub 2012 Jan 2. J Cell Biol. 2012. PMID: 22213802 Free PMC article. - A glycosphingolipid binding domain controls trafficking and activity of the mammalian notch ligand delta-like 1.
Heuss SF, Tarantino N, Fantini J, Ndiaye-Lobry D, Moretti J, Israël A, Logeat F. Heuss SF, et al. PLoS One. 2013 Sep 12;8(9):e74392. doi: 10.1371/journal.pone.0074392. eCollection 2013. PLoS One. 2013. PMID: 24069306 Free PMC article. - Neuralized promotes basal to apical transcytosis of delta in epithelial cells.
Benhra N, Vignaux F, Dussert A, Schweisguth F, Le Borgne R. Benhra N, et al. Mol Biol Cell. 2010 Jun 15;21(12):2078-86. doi: 10.1091/mbc.e09-11-0926. Epub 2010 Apr 21. Mol Biol Cell. 2010. PMID: 20410139 Free PMC article. - Drosophila Tempura, a novel protein prenyltransferase α subunit, regulates notch signaling via Rab1 and Rab11.
Charng WL, Yamamoto S, Jaiswal M, Bayat V, Xiong B, Zhang K, Sandoval H, David G, Gibbs S, Lu HC, Chen K, Giagtzoglou N, Bellen HJ. Charng WL, et al. PLoS Biol. 2014 Jan 28;12(1):e1001777. doi: 10.1371/journal.pbio.1001777. eCollection 2014 Jan. PLoS Biol. 2014. PMID: 24492843 Free PMC article.
References
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nature Rev. Mol. Cell Biol. 2006;7:678–689. - PubMed
- Schweisguth F. Regulation of Notch signaling activity. Curr. Biol. 2004;14:R129–R138. - PubMed
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nature Rev. 2006;7:93–102. - PubMed
- Bray S. Notch signalling in Drosophila: three ways to use a pathway. Sem. Cell Dev. Biol. 1998;9:591–597. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous