Rapid assessment of malaria transmission using age-specific sero-conversion rates - PubMed (original) (raw)
. 2009 Jun 29;4(6):e6083.
doi: 10.1371/journal.pone.0006083.
Roly Gosling, Jamie Griffin, Samwel Gesase, Joseph Campo, Ramadan Hashim, Paul Masika, Jacklin Mosha, Teun Bousema, Seif Shekalaghe, Jackie Cook, Patrick Corran, Azra Ghani, Eleanor M Riley, Chris Drakeley
Affiliations
- PMID: 19562032
- PMCID: PMC2698122
- DOI: 10.1371/journal.pone.0006083
Rapid assessment of malaria transmission using age-specific sero-conversion rates
Laveta Stewart et al. PLoS One. 2009.
Abstract
Background: Malaria transmission intensity is a crucial determinant of malarial disease burden and its measurement can help to define health priorities. Rapid, local estimates of transmission are required to focus resources better but current entomological and parasitological methods for estimating transmission intensity are limited in this respect. An alternative is determination of antimalarial antibody age-specific sero-prevalence to estimate sero-conversion rates (SCR), which have been shown to correlate with transmission intensity. This study evaluated SCR generated from samples collected from health facility attendees as a tool for a rapid assessment of malaria transmission intensity.
Methodology and principal findings: The study was conducted in north east Tanzania. Antibodies to Plasmodium falciparum merozoite antigens MSP-1(19) and AMA-1 were measured by indirect ELISA. Age-specific antibody prevalence was analysed using a catalytic conversion model based on maximum likelihood to generate SCR. A pilot study, conducted near Moshi, found SCRs for AMA-1 were highly comparable between samples collected from individuals in a conventional cross-sectional survey and those collected from attendees at a local health facility. For the main study, 3885 individuals attending village health facilities in Korogwe and Same districts were recruited. Both malaria parasite prevalence and sero-positivity were higher in Korogwe than in Same. MSP-1(19) and AMA-1 SCR rates for Korogwe villages ranged from 0.03 to 0.06 and 0.07 to 0.21 respectively. In Same district there was evidence of a recent reduction in transmission, with SCR among those born since 1998 [MSP-1(19) 0.002 to 0.008 and AMA-1 0.005 to 0.014 ] being 5 to 10 fold lower than among individuals born prior to 1998 [MSP-1(19) 0.02 to 0.04 and AMA-1 0.04 to 0.13]. Current health facility specific estimates of SCR showed good correlations with malaria incidence rates in infants in a contemporaneous clinical trial (MSP-1(19) r(2) = 0.78, p<0.01 & AMA-1 r(2) = 0.91, p<0.001).
Conclusions: SCRs generated from age-specific anti-malarial antibody prevalence data collected via health facility surveys were robust and credible. Analysis of SCR allowed detection of a recent drop in malaria transmission in line with recent data from other areas in the region. This health facility-based approach represents a potential tool for rapid assessment of recent trends in malaria transmission intensity, generating valuable data for local and national malaria control programs to target, monitor and evaluate their control strategies.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
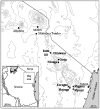
Figure 1. Map showing the study area: Squares mark major towns, circles mark villages with study dispensaries.
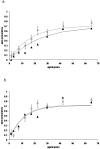
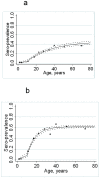
Figure 2. Age sero-prevalence plots for antibody responses to P. falciparum parasite antigens MSP-119 (fig 2a) and AMA-1 (figure 2b) from the pilot study (Msitu wa Tembo).
Open circles (and confidence limits) represent observed age group specific sero-prevalence points for the cross sectional survey. The dotted line represents a maximum likelihood fit using these data. The full triangles and unbroken line represent observed sero-prevalence points and fitted line for the health facility surveys.
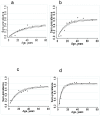
Figure 3. Age sero-prevalence plots for MSP-119 and AMA-1 fitted by maximum likelihood with a single force of infection for the dispensaries in the Kili-IPTi study.
Plot a) MSP-119 Same district; b) AMA-1 Same district; c) MSP-119 Korogwe district and d) AMA-1 Korogwe district. Black triangles represent observed data and black lines predicted values. Dotted black lines represent upper and lower 95% CI for the predicted SCR.
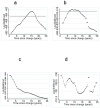
Figure 4. Univariate profile likelihood to evaluate the time at which sero-conversion rates changed.
Same region fits are represented in a) for MSP-119 fits and b) for AMA-1. The broken black line is the 95th percentile of the Chi-squared on 1 degree of freedom below the maximum. The two points at which this line crosses the log-likelihood profile are used to determine an approximate 95% confidence interval for the time since the change in SCR i.e. 11–18 years for MSP-119 and 6 to 14 years for AMA-1. The equivalent plots for Korogwe are shown in c) for MSP-119 and d) for AMA-1.
Figure 5. Age sero-prevalence plots for MSP-119(a) and AMA-1 (b) fitted by maximum likelihood with a two forces of infection for Same district.
Black triangles represent observed data and black lines predicted values. Dotted black lines represent upper and lower 95% CI for the predicted SCR.
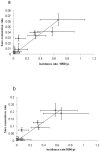
Figure 6. Current sero-conversion rates and clinical malaria incidence rates in the IPTi placebo cohort for each health facility: (a) for MSP-119 and (b) for AMA-1.
Vertical bars indicate the 95% CI for SCR and horizontal bars indicate the 95% CI for malaria incidence. Fitted lines represent linear regression plots. R2 values for MSP-119 and AMA are 0.78 and 0.91, respectively.
Similar articles
- Immunophoretic rapid diagnostic tests as a source of immunoglobulins for estimating malaria sero-prevalence and transmission intensity.
Williams GS, Mweya C, Stewart L, Mtove G, Reyburn H, Cook J, Corran PH, Riley EM, Drakeley CJ. Williams GS, et al. Malar J. 2009 Jul 22;8:168. doi: 10.1186/1475-2875-8-168. Malar J. 2009. PMID: 19624812 Free PMC article. - Serological markers for monitoring historical changes in malaria transmission intensity in a highly endemic region of Western Kenya, 1994-2009.
Wong J, Hamel MJ, Drakeley CJ, Kariuki S, Shi YP, Lal AA, Nahlen BL, Bloland PB, Lindblade KA, Were V, Otieno K, Otieno P, Odero C, Slutsker L, Vulule JM, Gimnig JE. Wong J, et al. Malar J. 2014 Nov 22;13:451. doi: 10.1186/1475-2875-13-451. Malar J. 2014. PMID: 25416454 Free PMC article. - Using serological measures to monitor changes in malaria transmission in Vanuatu.
Cook J, Reid H, Iavro J, Kuwahata M, Taleo G, Clements A, McCarthy J, Vallely A, Drakeley C. Cook J, et al. Malar J. 2010 Jun 16;9:169. doi: 10.1186/1475-2875-9-169. Malar J. 2010. PMID: 20553604 Free PMC article. - The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis.
Fowkes FJ, Richards JS, Simpson JA, Beeson JG. Fowkes FJ, et al. PLoS Med. 2010 Jan 19;7(1):e1000218. doi: 10.1371/journal.pmed.1000218. PLoS Med. 2010. PMID: 20098724 Free PMC article. Review. - Serology: a robust indicator of malaria transmission intensity?
Corran P, Coleman P, Riley E, Drakeley C. Corran P, et al. Trends Parasitol. 2007 Dec;23(12):575-82. doi: 10.1016/j.pt.2007.08.023. Epub 2007 Nov 7. Trends Parasitol. 2007. PMID: 17988945 Review.
Cited by
- Combined DNA extraction and antibody elution from filter papers for the assessment of malaria transmission intensity in epidemiological studies.
Baidjoe A, Stone W, Ploemen I, Shagari S, Grignard L, Osoti V, Makori E, Stevenson J, Kariuki S, Sutherland C, Sauerwein R, Cox J, Drakeley C, Bousema T. Baidjoe A, et al. Malar J. 2013 Aug 2;12:272. doi: 10.1186/1475-2875-12-272. Malar J. 2013. PMID: 23914905 Free PMC article. - Development of new malaria diagnostics: matching performance and need.
Bell D, Fleurent AE, Hegg MC, Boomgard JD, McConnico CC. Bell D, et al. Malar J. 2016 Aug 11;15(1):406. doi: 10.1186/s12936-016-1454-8. Malar J. 2016. PMID: 27515426 Free PMC article. - Influence of Landscape Patterns on Exposure to Lassa Fever Virus, Guinea.
Longet S, Leggio C, Bore JA, Key S, Tipton T, Hall Y, Koundouno FR, Bower H, Bhattacharyya T, Magassouba N, Günther S, Henao-Restrapo AM, Rossman JS, Konde MK, Fornace K, Carroll MW. Longet S, et al. Emerg Infect Dis. 2023 Feb;29(2):304-313. doi: 10.3201/eid2902.212525. Emerg Infect Dis. 2023. PMID: 36692336 Free PMC article. - Mosquito Bite-Induced Controlled Human Malaria Infection with Plasmodium vivax or P. falciparum Generates Immune Responses to Homologous and Heterologous Preerythrocytic and Erythrocytic Antigens.
Hall CE, Hagan LM, Bergmann-Leitner E, Tosh DM, Bennett JW, Regules JA, Chuang I, Angov E, Dutta S, Chattopadhyay D, Yadava A. Hall CE, et al. Infect Immun. 2019 Feb 21;87(3):e00541-18. doi: 10.1128/IAI.00541-18. Print 2019 Mar. Infect Immun. 2019. PMID: 30559218 Free PMC article. - Measuring changes in Plasmodium falciparum transmission: precision, accuracy and costs of metrics.
Tusting LS, Bousema T, Smith DL, Drakeley C. Tusting LS, et al. Adv Parasitol. 2014;84:151-208. doi: 10.1016/B978-0-12-800099-1.00003-X. Adv Parasitol. 2014. PMID: 24480314 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials