Maturation of active zone assembly by Drosophila Bruchpilot - PubMed (original) (raw)
Maturation of active zone assembly by Drosophila Bruchpilot
Wernher Fouquet et al. J Cell Biol. 2009.
Abstract
Synaptic vesicles fuse at active zone (AZ) membranes where Ca(2+) channels are clustered and that are typically decorated by electron-dense projections. Recently, mutants of the Drosophila melanogaster ERC/CAST family protein Bruchpilot (BRP) were shown to lack dense projections (T-bars) and to suffer from Ca(2+) channel-clustering defects. In this study, we used high resolution light microscopy, electron microscopy, and intravital imaging to analyze the function of BRP in AZ assembly. Consistent with truncated BRP variants forming shortened T-bars, we identify BRP as a direct T-bar component at the AZ center with its N terminus closer to the AZ membrane than its C terminus. In contrast, Drosophila Liprin-alpha, another AZ-organizing protein, precedes BRP during the assembly of newly forming AZs by several hours and surrounds the AZ center in few discrete punctae. BRP seems responsible for effectively clustering Ca(2+) channels beneath the T-bar density late in a protracted AZ formation process, potentially through a direct molecular interaction with intracellular Ca(2+) channel domains.
Figures
Figure 1.
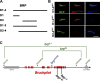
Epitope mapping for mAb Nc82 and genetic analysis of brp. (A) BRP fragments used for transgenic expression experiments. (B) Ectopic expression (in wing imaginal discs) of GFP-tagged BRP fragments missing either N- (D2-4) or C-terminal (D1-3) regions. Wing discs were costained for BRPN-Term (red), BRPNc82 (blue), and GFP (green). The D2-4 construct shows no BRPN-Term reactivity, whereas the D1-3 construct lacks BRPNc82 staining. Bar, 300 µm. (C) Genomic analysis of the brp locus. The deletion mutants (_brp_69 and _brp_6.1) and their parental transposon insert lines are shown in green, and the pBac transposon insert line is shown in blue. Position of EMS-induced stop codons of _brp_1.3 and _brp_5.45 are shown in black.
Figure 2.
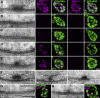
Combined electron and light microscopic analysis of AZ organization at NMJs of different brp alleles. (A–E, left) Ultrastructure of Drosophila NMJ AZs preserved with conventional room temperature embedding for transmission EM. The following genotypes are depicted: wild-type (wt; A), _brp_69 (B), _brp_c04298 (C), _brp_5.45 (D), and _brp_1.3 (E). (right) Corresponding confocal images of boutons costained for either BRPN-Term or BRPNc82 (magenta) and DGluRIIA or DGluRIID (green). (F) Wild-type and _brp_1.3 T-bars preserved using HPF followed by FS. For controls, long (left) and short axis (middle) views of T-bars are depicted; arrowheads indicate filaments emerging from the T-bar pedestal. (G and H, left) Conventionally embedded AZs after expression of BRPD1-3GFP and BRPD2-4GFP in the _brp_69 mutant background. In BRPD1-3GFP, T-bar formation could not be observed. For BRPD2-4GFP, electron-dense structures much smaller than T-bars were observed. (right) Corresponding confocal images of the reexpression constructs costained for GFP (magenta) and DGluRIID (green). Bars: (G) 200 nm; (E and H) 500 nm.
Figure 3.
Immuno-EM versus two BRP epitopes at Drosophila NMJ AZs. (A) High pressure frozen bouton prepared for immunogold labeling, indicating a T-bar labeled for BRPN-Term (arrowhead). (B and C) Magnifications of individual planar (left) and vertically (right) imaged T-bars labeled (after embedding) for either BRPN-Term (B) or BRPNc82 (C). Gold particles are highlighted by red circles. (D) Quantification of BRPN-Term and BRPNc82 signals of the closest distance of individual gold particles to the AZ membrane. Error bars indicate mean ± SEM. ***, P < 0.005. Bars: (A) 150 nm; (B) 200 nm.
Figure 4.
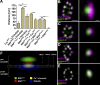
Polarized orientation of BRP at AZs. (A) Distances of center to center intensity maxima for different synaptic labels are as follows: CacGFP × DGluRIID, 37.9 ± 11.9 nm (n = 30); BRPNc82 × DGluRIID, 155.2 ± 5.7 nm (n = 30); BRPN-Term × DGluRIIA, 93.4 ± 11.3 nm (n = 30); BRPNc82 × CacGFP, 109.8 ± 4.6 nm (n = 30); BRPN-Term × CacGFP, 65.6 ± 7.9 nm (n = 30); BRPNc82 × BRPN-Term, 67.5 ± 3.8 nm (n = 70); D1-4GFP × BRPN-Term, 64.2 ± 5.1 nm (n = 40); BRPNc82 × BRPNc82, 0.0 ± 5.2 nm (n = 30). Error bars indicate mean ± SEM. **, P < 0.01; ***, P < 0.005. (B–E) Confocal images of midsections through the bouton (left) and single vertically imaged synapses (right) with the bouton lumen facing left. (B) Magenta, DGluRIID; green, BRPNc82. Bars: 500 nm (left) and 100 nm (right). (C) Magenta, CacGFP; green, BRPNc82. (D) Magenta, BRPN-Term; green, BRPNc82. (E) Magenta, BRPNc82; green, BRPNc82. (F) Schematic model of protein epitope distribution at an individual NMJ AZ.
Figure 5.
STED analysis of AZ organization at Drosophila NMJ synapses. (A) Overview of a bouton stained for BRPN-Term (confocal; magenta) and BRPNc82 (STED; green) showing planar (arrow) and vertical (arrowhead) AZs. (B and C) Magnifications of individual planar (left) and vertical (right) AZs stained for BRPNc82 (STED) and BRPN-Term (confocal; B) and BRPN-Term (STED) and CacGFP (confocal; C). (D) Mean normalized planar BRPN-Term (magenta) and BRPNc82 (green) arrangement shown with STED resolution (BRPN-Term, n = 14; BRPNc82, n = 47). (right) The merge superimposed with the intensity profile along one axis through the midpoint for BRPN-Term (magenta) and BRPNc82 (green) is shown. Error bars indicate ± SEM. (E) BRPNc82 (STED) and BRPN-Term (confocal) after expression of full-length BRP cDNA in _brp_69 background (BRPD1-4). (F and G) Individual planar (left) and vertical (right) AZs stained for BRPNc82 (STED) and CacGFP (confocal; F) and CacGFP (STED) and BRPNc82 (confocal; G). All images were deconvolved using Imspector software. Bars: (A) 1 µm; (G) 100 nm.
Figure 6.
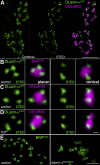
STED microscopic analysis of DLiprin-αGFP at AZs. (A) Single confocal sections of NMJs colabeled for DLiprin-αGFP (green, confocal resolution in left image and STED resolution in middle image) and DGluRIID (magenta, confocal overlay in right image). STED images of DLiprin-αGFP reveal substructures beyond the diffraction limit of confocal microscopy. (B–D) STED images of an individual AZ. Discrete dots of DLiprin-αGFP are arranged at the AZ edge (magenta, BRPNc82 [B] and DGluRIID [C and D]). Left, planar AZ; right, vertical AZ. B and C show controls, and D shows _brp_69. (E) Single confocal slices of control (left) and dliprin-α (right) junctions labeled for BRPNc82 with STED resolution. Atypical clusters of BRP doughnuts are observed at dliprin-α mutant NMJs. Bars: (A) 1.5 µm; (D) 100 nm; (E) 1 µm.
Figure 7.
In vivo analysis of synaptic protein accumulation. (A–E) Confocal stacks of sequentially in vivo–imaged NMJs (muscle 26) at Δt = 12 h. NMJs coexpress the indicated labels (green, GFP constructs; magenta, mRFP constructs). (top) Individual in vivo–imaged synapses (arrowheads) positive for only one label at t = 0 h but positive for both labels at t = 12 h are shown. (bottom) A prospective synapse (arrows) positive for only one label at t = 12 h is shown. Bars, 1 µm.
Figure 8.
Size-dependent Ca2+ channel–clustering defects in brp. (A) CacGFP (green) clustering at AZs (identified at AZs opposite DGluRIID (magenta)) in control, _brp_6.1, _brp_69, _brp_c04298, and _brp_1.3 animals. (B) Comparison of Ca2+ channel clustering at control and _brp_c04298 NMJs expressing CacGFP and DGluRIIAmRFP. Small PSDs in brp mutants opposite CacGFP clusters of comparable intensity to controls (arrowheads). Larger PSDs, indicating a more advanced synaptic maturation state, typically display severely decreased CacGFP intensity values compared with controls (arrows). (C) Statistical analysis shows no significant differences in the maximum CacGFP intensity opposite particularly small PSDs (control: 0–0.118 µm2 = 76.7 ± 4.4 au [n = 66]; 0.119–0.356 µm2 = 113.0 ± 2.3 au [n = 161]; 0.357–0.715 µm2 = 1,41.0 ± 4.0 au [n = 46]; _brp_c04298: 0–0.118 µm2 = 66.0 ± 3.9 au [n = 34]; 0.119–0.356 µm2 = 83.7 ± 1.6 au [n = 157]; 0.357–0.715 µm2 = 91.4 ± 2.1 au [n = 73]; P = 0.12, P < 0.005, and P < 0.005 by Student’s t test). (D) FRAP experiment for CacGFP with a recovery interval of 12 h. FRAP was comparable between _brp_69 and control boutons (control: 0.47 ± 0.04 [n = 6]; _brp_69: 0.38 ± 0.04 [n = 6]; P = 0.3 by Mann-Whitney test). (E) Costaining of BRPN-Term (magenta) and CacGFP (green) at _brp_1.3 boutons and controls. Those AZs showing restored Cac clustering also show restored BRPN-Term label (arrowheads). (F) Ultrastructure of the area between presynaptic membrane and the T-bar pedestal (arrows) prepared via HPF/FS. Asterisks indicate the synaptic cleft. At control (top) and _brp_1.3 (middle) AZs, discrete regularly arranged elements emerging from the AZ membrane (∼5–7 nm; arrowheads) are observed within the gap between the AZ membrane and the T-bar pedestal. These elements are not observed at _brp_69 AZs lacking T-bars (bottom). Error bars indicate mean ± SEM. ***, P < 0.005. Bars: (E) 1 µm; (F) 25 nm.
Figure 9.
The N terminus of BRP physically interacts with the C terminus of Cac in vitro. (A) Scheme of yeast two-hybrid analysis using Cac bait constructs and overlapping BRP prey constructs. Full-length Cac protein comprises three large intracellular loops (aa are indicated) and its intracellular C-terminal region (aa 1,420–1,848). The N terminus of BRP (aa 1–320) interacts with the C-terminal region of Cac (83% of the reported cytoplasmic C terminus, sparing the EF hand and most of its IQ motif; Kawasaki et al., 2002). −, no interaction; +++, interaction of high confidence. (B) Co-IPs from Schneider cell extracts cotransfected with a GFP-tagged N-terminal construct of BRP (D1-2GFP; aa 1–617) and Myc-tagged CacC-Term. Western blotting shows the pull-down of the BRP construct in the anti-Myc IP at ∼100 kD (arrow). The corresponding band is not detected in the control lanes (IgGs). The slightly different migration of the band in the input lane and in the IP lanes is the result of differences in sample buffer. MW, molecular weight. (C) Spatiotemporal model of AZ assembly and organization at Drosophila NMJs. SVs, synaptic vesicles.
Similar articles
- Structural Remodeling of Active Zones Is Associated with Synaptic Homeostasis.
Hong H, Zhao K, Huang S, Huang S, Yao A, Jiang Y, Sigrist S, Zhao L, Zhang YQ. Hong H, et al. J Neurosci. 2020 Apr 1;40(14):2817-2827. doi: 10.1523/JNEUROSCI.2002-19.2020. Epub 2020 Mar 2. J Neurosci. 2020. PMID: 32122953 Free PMC article. - A Syd-1 homologue regulates pre- and postsynaptic maturation in Drosophila.
Owald D, Fouquet W, Schmidt M, Wichmann C, Mertel S, Depner H, Christiansen F, Zube C, Quentin C, Körner J, Urlaub H, Mechtler K, Sigrist SJ. Owald D, et al. J Cell Biol. 2010 Feb 22;188(4):565-79. doi: 10.1083/jcb.200908055. J Cell Biol. 2010. PMID: 20176924 Free PMC article. - Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila.
Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Dürrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, Wichmann C, Kittel R, Sigrist SJ, Buchner E. Wagh DA, et al. Neuron. 2006 Mar 16;49(6):833-44. doi: 10.1016/j.neuron.2006.02.008. Neuron. 2006. PMID: 16543132 - The active zone T-bar--a plasticity module?
Wichmann C, Sigrist SJ. Wichmann C, et al. J Neurogenet. 2010 Sep;24(3):133-45. doi: 10.3109/01677063.2010.489626. J Neurogenet. 2010. PMID: 20553221 Review. - Synaptic homeostasis on the fast track.
Macleod GT, Zinsmaier KE. Macleod GT, et al. Neuron. 2006 Nov 22;52(4):569-71. doi: 10.1016/j.neuron.2006.11.006. Neuron. 2006. PMID: 17114040 Review.
Cited by
- Presynaptic active zones in invertebrates and vertebrates.
Ackermann F, Waites CL, Garner CC. Ackermann F, et al. EMBO Rep. 2015 Aug;16(8):923-38. doi: 10.15252/embr.201540434. Epub 2015 Jul 9. EMBO Rep. 2015. PMID: 26160654 Free PMC article. Review. - Synaptic components are required for glioblastoma progression in Drosophila.
Losada-Pérez M, Hernández García-Moreno M, García-Ricote I, Casas-Tintó S. Losada-Pérez M, et al. PLoS Genet. 2022 Jul 25;18(7):e1010329. doi: 10.1371/journal.pgen.1010329. eCollection 2022 Jul. PLoS Genet. 2022. PMID: 35877760 Free PMC article. - Complexin cooperates with Bruchpilot to tether synaptic vesicles to the active zone cytomatrix.
Scholz N, Ehmann N, Sachidanandan D, Imig C, Cooper BH, Jahn O, Reim K, Brose N, Meyer J, Lamberty M, Altrichter S, Bormann A, Hallermann S, Pauli M, Heckmann M, Stigloher C, Langenhan T, Kittel RJ. Scholz N, et al. J Cell Biol. 2019 Mar 4;218(3):1011-1026. doi: 10.1083/jcb.201806155. Epub 2019 Feb 19. J Cell Biol. 2019. PMID: 30782781 Free PMC article. - Rapid active zone remodeling during synaptic plasticity.
Weyhersmüller A, Hallermann S, Wagner N, Eilers J. Weyhersmüller A, et al. J Neurosci. 2011 Apr 20;31(16):6041-52. doi: 10.1523/JNEUROSCI.6698-10.2011. J Neurosci. 2011. PMID: 21508229 Free PMC article. - Structural Remodeling of Active Zones Is Associated with Synaptic Homeostasis.
Hong H, Zhao K, Huang S, Huang S, Yao A, Jiang Y, Sigrist S, Zhao L, Zhang YQ. Hong H, et al. J Neurosci. 2020 Apr 1;40(14):2817-2827. doi: 10.1523/JNEUROSCI.2002-19.2020. Epub 2020 Mar 2. J Neurosci. 2020. PMID: 32122953 Free PMC article.
References
- Cao Y.Q., Piedras-Renteria E.S., Smith G.B., Chen G., Harata N.C., Tsien R.W. 2004. Presynaptic Ca2+ channels compete for channel type-preferring slots in altered neurotransmission arising from Ca2+ channelopathy.Neuron. 43:387–400 - PubMed
- Catterall W.A. 1998. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release.Cell Calcium. 24:307–323 - PubMed
- Catterall W.A., Few A.P. 2008. Calcium channel regulation and presynaptic plasticity.Neuron. 59:882–901 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous