The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: knowledge from the 50/10 OIR model - PubMed (original) (raw)
Review
The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: knowledge from the 50/10 OIR model
M Elizabeth Hartnett. Doc Ophthalmol. 2010 Feb.
Abstract
The objective of this study is to determine growth factor expression and activation of signaling pathways associated with intravitreous neovascularization and peripheral avascular retina using a model of retinopathy of prematurity (ROP) relevant to today with oxygen monitoring in neonatal units. Studies using 50/10 oxygen-induced retinopathy (OIR) and 50/10 OIR+SO models were reviewed. Repeated fluctuations in oxygen increased retinal vascular endothelial growth factor (VEGF) even while peripheral avascular retina persisted and prior to the development of intravitreous neovascularization. Repeated fluctuations in oxygen increased VEGF(164) expression but not VEGF(120). Neutralizing VEGF bioactivity significantly reduced intravitreous neovascularization and arteriolar tortuosity without interfering with ongoing retinal vascularization. Repeated oxygen fluctuations led to retinal hypoxia and increased reactive oxygen species (ROS). Inhibiting ROS with NADPH oxidase inhibitor, apocynin, reduced avascular retina by interfering with apoptosis. Supplemental oxygen reduced retinal VEGF concentration and exacerbated NADPH oxidase activation to contribute to intravitreous neovascularization through activation of the JAK/STAT pathway. Oxygen stresses relevant to those experienced by preterm infants today trigger signaling of different pathways to cause avascular retina and intravitreous neovascularization. Increased signaling of VEGF appears important to the development of both avascular retina and intravitreous neovascularization.
Figures
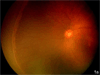
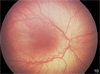
Figure 1
a. Zone II with ridge in the nasal periphery of the left eye of a preterm infant showing early stage 3 ROP, or intravitreous neovascularzation (Retcam image, Clarity, CA). b. Left eye of preterm infant with stage 3 ROP (intravitreous neovascularization) showing flat neovascularization that can occur in zone I and posterior zone II ROP. (see inset zoomed image; Retcam image, Clarity, CA).
Figure 1
a. Zone II with ridge in the nasal periphery of the left eye of a preterm infant showing early stage 3 ROP, or intravitreous neovascularzation (Retcam image, Clarity, CA). b. Left eye of preterm infant with stage 3 ROP (intravitreous neovascularization) showing flat neovascularization that can occur in zone I and posterior zone II ROP. (see inset zoomed image; Retcam image, Clarity, CA).
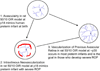
Figure 2
50/10 OIR model at different post-natal day ages (p) mimics what occurs in human preterm infants. (1) at birth, human preterm infants have incompletely vascularized retina; (2) approximately 6% of preterm infants born less than 1000 grams in birth weight will develop severe ROP, at which time treatment with laser is recommended to reduce unwanted intravitreous neovascularization and consequences of retinal detachment; (3) most preterm infants will undergo vascularization of the avascular retina and 50% of infants with threshold severe ROP will also have regression of the intravitreous neovascularization with ongoing vascularization of the avascular retina. The goal of our lab is to promote vascularization of avascular retina and to reduce unwanted intravitreous neovascularization.
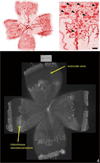
Figure 3
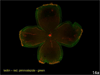
A. Lectin-stained retinal flat mount of pup in 50/10 OIR model at postnatal day (p)14 after 7 cycles of oxygen fluctuations between 50% and 10% inspired oxygen showing peripheral avascular retina, similar to that in zone II ROP. B. At p18, after return to room air for 4 additional days, intravitreous neovascularization develops at the junction of vascular and peripheral avascular retina.
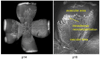
Figure 4
(top). Lectin-stained retinal flat mount of mouse model of hyperoxia induced vaso-obliteration and angiogenesis at p17 following 5 days of 75% oxygen and 5 days of room air showing central obliteration of retinal capillaries with endothelial budding (model developed by Lois Smith). (bottom). Lectin-stained retinal flat mount of rat 50/10 model of oxygen induced retinopathy following 7 cycles of oxygen between 50% and 10%, then 4 days of room air exposure, showing peripheral avascular retina and intravitreous neovascularization at the junction of vascular and avascular retina.
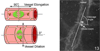
Figure 5
Graph of inspired oxygen over time in the 50/10 OIR developed by John Penn and 50/10 OIR+SO models. At postnatal day 14 pups are returned to room air (50/10 OIR) or recovered in supplemental oxygen (28% oxygen, 50/10 OIR+SO) models. The models have relevance to human ROP in that the arterial oxygen of pups in these oxygen extremes mimic the transcutaneous oxygen extremes in human preterm infants that develop severe ROP. Also fluctuations in oxygen are risks for ROP. The model also develops features similar to infants with severe ROP of today.
Figure 6
Lectin-stained retinal flat mount of pup in 50/10 OIR model at postnatal day (p)18 after 7 cycles of oxygen fluctuations between 50% and 10% inspired oxygen and return to room air for 4 additional days showing what features can be quantified reproducibly in the model (avascular/total retinal area, clock hours or area of intravitreous neovascularization, tortuosity of retinal vessels, capillary density within vascularized retina).
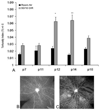
Figure 7
Effect of a single episode of hypoxia on VEGF isoform expression. A: RT-PCR of VEGF isoform mRNAs in P7 (lane 1) and P14 (lane 3) control animals in room air (RA), and animals that had a single hypoxic episode of 24 h of 10% oxygen (10% O2) at either P7 (lane 2) or P14 (lane 4). PCR was repeated three times on different experiments and this result is representative. B: Densitometry measurements using 18S RNA as the control gene. Samples were assayed in triplicate and error bars are standard deviations. An asterisk (“*”) indicates p<0.01 compared to respective controls, t test. (permission from Molecular Vision 2004; 10:512–20,
http://www.molvis.org/molvis/v10/a63
).
Figure 8
a. VEGF retinal protein from room air raised and 50/10 OIR exposed pups at different postnatal day ages by ELISA and showing increase in VEGF in retinas prior to development of intravitreous neovascularization and compared to room air b. At p18, retinal VEGF was 10-fold that in the vitreous. (permission from Molecular Vision 2008; 14:345–357,
http://www.molvis.org/molvis/v14/a43
).
Figure 8
a. VEGF retinal protein from room air raised and 50/10 OIR exposed pups at different postnatal day ages by ELISA and showing increase in VEGF in retinas prior to development of intravitreous neovascularization and compared to room air b. At p18, retinal VEGF was 10-fold that in the vitreous. (permission from Molecular Vision 2008; 14:345–357,
http://www.molvis.org/molvis/v14/a43
).
Figure 9
Clock hours of intravitreous neovascularization and avascular/total retinal areas of lectin-stained retinal flat mounts from rat pups in 50/10 oxygen induced retinopathy after intravitreous injection of neutralizing antibody to vascular endothelial growth factor (VEGFab) or control nonimmune rat IgG. A: Mean clock hours of intravitreous neovascularization (IVNV)at p18 were significantly decreased by injection of either 25 ng or 50 ng VEGFab compared to IgG control (ANOVA p<0.001; * posthoc Student’s _t_-tests p<0.001 compared to IgG). B: Mean clock hours of IVNV at p25 were significantly decreased by injection of 50 ng VEGFab compared to IgG control (ANOVA p=0.003; * posthoc Student’s _t_-test p=0.003). C: Peripheral avascular/total retinal area as no different in retinas treated with either VEGFab dose compared to IgG control at p18 (ANOVA p=0.238). D: At p25, the overall ANOVA for peripheral avascular/total retinal area was significant (p=0.038). Posthoc testing showed the 50 ng dose of VEGFab was significantly decreased compared to the 25 ng dose (* posthoc Student’s _t_-test, 25 ng versus 50 ng, p=0.038). However, neither dose of VEGFab was significantly different to control IgG. (permission from _Molecular Vision_2008; 14:345–357,
http://www.molvis.org/molvis/v14/a43
).
Figure 10
Retcam image (Clarity, CA) of right eye of human preterm infant demonstrating dilated and tortuous vessels seen in plus disease, a feature of severe ROP.
Figure 11
A. Tortuosity indices in retinal arterioles measured in rats raised in room air or 50/10 OIR model at different postnatal day (p) ages showing increased tortuosity at p12 and p14. Bottom: Representative lectin-stained retinal flat mount at p14 in room air (B) and 50/10 OIR (C) models. (re-plotted from Investigative Ophthalmology and Visual Sciences; 2008 Jul;49(7):3107–14).
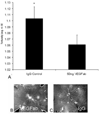
Figure 12
Arteriolar tortuosity index of retinas from eyes injected with either 50 ng VEGF antibody (VEGFab) or 50 ng control IgG at p12 and analyzed at p14. (A) The tortuosity index was significantly reduced in eyes injected with 50 ng VEGFab compared with that in IgG-injected eyes (ANOVA *P = 0.004, post hoc protected _t_-test). (B) Examples of retinas from eyes injected with 50 ng VEGFab and (C) 50 ng control IgG resulted in tortuosity indices of 1.03 ±0.007 and 1.11 ±0.04, respectively. (re-plotted from Investigative Ophthalmology and Visual Sciences; 2008 Jul;49(7):3107–14).
Figure 13
Left: Artist diagram showing long axis of vessel and cleavage plane angle. An angle of 90 degrees predicts elongation of the vessel based on the migration of the dividing endothelial cells whereas an angle of 180 degrees predicts dilation. An irregular group of cleavage plane angles may be seen in disordered angiogenesis. Right: Lectin stained flat mounted vessel with cleavage planes drawn in to demonstrate how technique is performed.
Figure 14
a. Lectin stained retinal flat mount of room air raised postnatal day (p)4 rat pup injected with pimonidazole 90 minutes before sacrifice. Lectin stains retina vessels (red) and insoluble pimonidazole conjugates (green) are present in tissue where oxygen concentration is less than 10 mm Hg. Despite nearly 50% of the retina as avascular, little insoluble pimonidazole is present. b. Lectin stained retinal flat mount of postnatal day (p)18 rat pup from the 50/10 OIR model injected with pimonidazole 90 minutes before sacrifice. Lectin stains retina vessels (red) and insoluble pimonidazole conjugates (green) are present in tissue where oxygen concentration is less than 10 mm Hg. Hypoxic retina (green labeling conjugated pimonidazole) is present in avascular retina and in areas in between vessels within vascularized retina.
Figure 14
a. Lectin stained retinal flat mount of room air raised postnatal day (p)4 rat pup injected with pimonidazole 90 minutes before sacrifice. Lectin stains retina vessels (red) and insoluble pimonidazole conjugates (green) are present in tissue where oxygen concentration is less than 10 mm Hg. Despite nearly 50% of the retina as avascular, little insoluble pimonidazole is present. b. Lectin stained retinal flat mount of postnatal day (p)18 rat pup from the 50/10 OIR model injected with pimonidazole 90 minutes before sacrifice. Lectin stains retina vessels (red) and insoluble pimonidazole conjugates (green) are present in tissue where oxygen concentration is less than 10 mm Hg. Hypoxic retina (green labeling conjugated pimonidazole) is present in avascular retina and in areas in between vessels within vascularized retina.
Figure 15
a. Lipid hydroperoxide in 50/10 oxygen-induced retinopathy model. Lipid hydroperoxide (LHP) levels in retinas assayed at postnatal days 7, 14, or 18 (p7, p14, p18) from pups injected intraperitoneally (IP) with n-acetylcysteine (NAC) or PBS at postnatal days p2, p6, and p10 in the 50/10 oxygen-induced retinopathy (OIR) model. Overall ANOVA, p<0.001, * p=0.012, Bonferroni post-hoc analysis comparing p18 NAC to p18 PBS injected. (n=8 animals at p7 and 4 animals at p14 and p18). b. Apocynin treatment significantly reduced percent avascular retina at p18 compared to PBS control. Avascular retinal area and clock hours of intravitreous neovascularization and capillary densities of apocynin treated pups. A: Percent avascular retinal area in 50/10 OIR model after intraperitoneal (IP) injections with apocynin once every 24 h from p12 to p17 and assayed at p18 was significantly reduced compared to phosphate buffered saline (PBS) injected pups (* p=0.017, Student’s t-test; n=11 retinas each). B: Clock hours of intravitreous neovascularization (IVNV) in retinas from 50/10 OIR model after IP injections with apocynin once every 24 h from p12 to p17 and assayed at p18 were not significantly different to PBS injected pups (p=0.26; Mann- Whitney test, n=11). c. Cleaved caspase-3 expression in the retinas of 50/10 OIR after IP injection with apocynin on p12 and p13 and assayed on p14 was significantly decreased compared to retinas from PBS injected pups (p=0.021, Student’s t-test, n=4). However, no significant difference in cleaved caspase-3 expression was measured in retinas of pups injected with apocynin every 24 h from p12 to 17 and assayed on p18 compared to PBS injected pups (p=0.78, Student t-test, data not shown). (permission from _Molecular Vision_2007; 13:840–53
http://www.molvis.org/molvis/v13/a92/
)
Figure 15
a. Lipid hydroperoxide in 50/10 oxygen-induced retinopathy model. Lipid hydroperoxide (LHP) levels in retinas assayed at postnatal days 7, 14, or 18 (p7, p14, p18) from pups injected intraperitoneally (IP) with n-acetylcysteine (NAC) or PBS at postnatal days p2, p6, and p10 in the 50/10 oxygen-induced retinopathy (OIR) model. Overall ANOVA, p<0.001, * p=0.012, Bonferroni post-hoc analysis comparing p18 NAC to p18 PBS injected. (n=8 animals at p7 and 4 animals at p14 and p18). b. Apocynin treatment significantly reduced percent avascular retina at p18 compared to PBS control. Avascular retinal area and clock hours of intravitreous neovascularization and capillary densities of apocynin treated pups. A: Percent avascular retinal area in 50/10 OIR model after intraperitoneal (IP) injections with apocynin once every 24 h from p12 to p17 and assayed at p18 was significantly reduced compared to phosphate buffered saline (PBS) injected pups (* p=0.017, Student’s t-test; n=11 retinas each). B: Clock hours of intravitreous neovascularization (IVNV) in retinas from 50/10 OIR model after IP injections with apocynin once every 24 h from p12 to p17 and assayed at p18 were not significantly different to PBS injected pups (p=0.26; Mann- Whitney test, n=11). c. Cleaved caspase-3 expression in the retinas of 50/10 OIR after IP injection with apocynin on p12 and p13 and assayed on p14 was significantly decreased compared to retinas from PBS injected pups (p=0.021, Student’s t-test, n=4). However, no significant difference in cleaved caspase-3 expression was measured in retinas of pups injected with apocynin every 24 h from p12 to 17 and assayed on p18 compared to PBS injected pups (p=0.78, Student t-test, data not shown). (permission from _Molecular Vision_2007; 13:840–53
http://www.molvis.org/molvis/v13/a92/
)
Figure 15
a. Lipid hydroperoxide in 50/10 oxygen-induced retinopathy model. Lipid hydroperoxide (LHP) levels in retinas assayed at postnatal days 7, 14, or 18 (p7, p14, p18) from pups injected intraperitoneally (IP) with n-acetylcysteine (NAC) or PBS at postnatal days p2, p6, and p10 in the 50/10 oxygen-induced retinopathy (OIR) model. Overall ANOVA, p<0.001, * p=0.012, Bonferroni post-hoc analysis comparing p18 NAC to p18 PBS injected. (n=8 animals at p7 and 4 animals at p14 and p18). b. Apocynin treatment significantly reduced percent avascular retina at p18 compared to PBS control. Avascular retinal area and clock hours of intravitreous neovascularization and capillary densities of apocynin treated pups. A: Percent avascular retinal area in 50/10 OIR model after intraperitoneal (IP) injections with apocynin once every 24 h from p12 to p17 and assayed at p18 was significantly reduced compared to phosphate buffered saline (PBS) injected pups (* p=0.017, Student’s t-test; n=11 retinas each). B: Clock hours of intravitreous neovascularization (IVNV) in retinas from 50/10 OIR model after IP injections with apocynin once every 24 h from p12 to p17 and assayed at p18 were not significantly different to PBS injected pups (p=0.26; Mann- Whitney test, n=11). c. Cleaved caspase-3 expression in the retinas of 50/10 OIR after IP injection with apocynin on p12 and p13 and assayed on p14 was significantly decreased compared to retinas from PBS injected pups (p=0.021, Student’s t-test, n=4). However, no significant difference in cleaved caspase-3 expression was measured in retinas of pups injected with apocynin every 24 h from p12 to 17 and assayed on p18 compared to PBS injected pups (p=0.78, Student t-test, data not shown). (permission from _Molecular Vision_2007; 13:840–53
http://www.molvis.org/molvis/v13/a92/
)
Figure 16
ELISA of VEGF from pups in 50/10 OIR or in 50/10 OIR+SO models treated with either IP injections of apocynin (10mg/kg/day) or equivalent volume of PBS from p12-p17 and assayed at p18. Student’s t-test. (re-plotted from Investigative Ophthalmology and Visual Sciences, 2008; 49(4);1591–1598.)
Figure 17
a. Phosphorylated p47-phox, a subunit of activated NADPH oxidase, is increased in the 50/10 OIR+SO model compared to 50/10 OIR model. b. Inhibition of ROS from activated NADPH oxidase using apocynin reduces area of intravitreous neovascularization in the 50/10 OIR+SO model. (re-plotted from Investigative Ophthalmology and Visual Sciences, 2008; 49(4);1591–1598.)
Figure 17
a. Phosphorylated p47-phox, a subunit of activated NADPH oxidase, is increased in the 50/10 OIR+SO model compared to 50/10 OIR model. b. Inhibition of ROS from activated NADPH oxidase using apocynin reduces area of intravitreous neovascularization in the 50/10 OIR+SO model. (re-plotted from Investigative Ophthalmology and Visual Sciences, 2008; 49(4);1591–1598.)
Figure 18
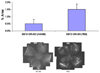
Areas of intravitreous neovascularization (IVNV) at postnatal day (p)18 in retinas from the 50/10 OIR+SO model treated with daily intraperitoneal (IP) injections (5mg/kg) of AG490 or PBS starting at p3 and continuing through p17. Top: Mean pixel density in 10 or more retinas analyzed per group, *p=0.03. Bottom: Flat mount image representative of each treatment group. (re-plotted from Investigative Ophthalmology and Visual Sciences, 2009; in press.)
Similar articles
- The role of supplemental oxygen and JAK/STAT signaling in intravitreous neovascularization in a ROP rat model.
Byfield G, Budd S, Hartnett ME. Byfield G, et al. Invest Ophthalmol Vis Sci. 2009 Jul;50(7):3360-5. doi: 10.1167/iovs.08-3256. Epub 2009 Mar 5. Invest Ophthalmol Vis Sci. 2009. PMID: 19264880 Free PMC article. - Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model.
Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Saito Y, et al. Invest Ophthalmol Vis Sci. 2008 Apr;49(4):1591-8. doi: 10.1167/iovs.07-1356. Invest Ophthalmol Vis Sci. 2008. PMID: 18385079 Free PMC article. - Association of retinal vascular endothelial growth factor with avascular retina in a rat model of retinopathy of prematurity.
Budd SJ, Thompson H, Hartnett ME. Budd SJ, et al. Arch Ophthalmol. 2010 Aug;128(8):1014-21. doi: 10.1001/archophthalmol.2010.158. Arch Ophthalmol. 2010. PMID: 20697002 Free PMC article. - Retinal vascular development and oxygen-induced retinopathy: a role for adenosine.
Lutty GA, McLeod DS. Lutty GA, et al. Prog Retin Eye Res. 2003 Jan;22(1):95-111. doi: 10.1016/s1350-9462(02)00058-7. Prog Retin Eye Res. 2003. PMID: 12597925 Review.
Cited by
- Clinical Efficacy of Topical CoQ10 and Vitamin-E Eye-drop in Retinopathy of Prematurity.
Akdogan M, Polat O. Akdogan M, et al. Med Hypothesis Discov Innov Ophthalmol. 2019 Winter;8(4):291-297. Epub 2019 Oct 1. Med Hypothesis Discov Innov Ophthalmol. 2019. PMID: 31788491 Free PMC article. - 17-Alpha-estradiol ameliorating oxygen-induced retinopathy in a murine model.
Zhang HB, Sun NX, Liang HC, Xiao XH, Liu XN, Wang YN. Zhang HB, et al. Jpn J Ophthalmol. 2012 Jul;56(4):407-15. doi: 10.1007/s10384-012-0136-5. Epub 2012 May 15. Jpn J Ophthalmol. 2012. PMID: 22581453 - Retinal VEGF levels correlate with ocular circulation measured by a laser speckle-micro system in an oxygen-induced retinopathy rat model.
Matsumoto T, Saito Y, Itokawa T, Shiba T, Oba MS, Takahashi H, Hori Y. Matsumoto T, et al. Graefes Arch Clin Exp Ophthalmol. 2017 Oct;255(10):1981-1990. doi: 10.1007/s00417-017-3756-0. Epub 2017 Aug 8. Graefes Arch Clin Exp Ophthalmol. 2017. PMID: 28791491 - Apocynin protects endothelial cells from endoplasmic reticulum stress-induced apoptosis via IRE1α engagement.
Wu J, Zhang W, Liu X, Wu L, He G, Li P, Guo X, Chen Z, Huang Q. Wu J, et al. Mol Cell Biochem. 2018 Dec;449(1-2):257-265. doi: 10.1007/s11010-018-3362-4. Epub 2018 Apr 25. Mol Cell Biochem. 2018. PMID: 29696609 Free PMC article. - Deactivation of the rod response in retinopathy of prematurity.
Hansen RM, Harris ME, Moskowitz A, Fulton AB. Hansen RM, et al. Doc Ophthalmol. 2010 Aug;121(1):29-35. doi: 10.1007/s10633-010-9228-z. Epub 2010 Mar 27. Doc Ophthalmol. 2010. PMID: 20349203 Free PMC article.
References
- Berkowitz BA, Zhang W. Significant reduction of the panretinal oxygenation response after 28% supplemental oxygen recovery in experimental ROP. Invest Ophthalmol Vis Sci. 2000;41:1925–1931. - PubMed
- McColm JR, Hartnett ME. Pediatric Retinal Diseases: Medical and Surgical Approaches. Philadelphia: Lippincot Williams & Wilkins; 2005. Retinopathy of prematurity: current understanding based on clinical trials and animal models; pp. 387–409.
- Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity Ophthalmological Outcomes at 10 Years. Arch Ophthalmol. 2001;119:1110–1118. - PubMed
- Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1694. - PubMed
- Flynn JT. Retinopathy of prematurity. Ped Clin N Am. 1987;34:1487–1516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 EY015130/EY/NEI NIH HHS/United States
- R01 EY015130/EY/NEI NIH HHS/United States
- EY015130/EY/NEI NIH HHS/United States
- EY017011/EY/NEI NIH HHS/United States
- R01 EY017011/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources