RAKing in AKT: a tumor suppressor function for the intracellular tyrosine kinase FRK - PubMed (original) (raw)
Review
. 2009 Sep 1;8(17):2728-32.
doi: 10.4161/cc.8.17.9389. Epub 2009 Sep 29.
Affiliations
- PMID: 19652529
- PMCID: PMC3005195
- DOI: 10.4161/cc.8.17.9389
Review
RAKing in AKT: a tumor suppressor function for the intracellular tyrosine kinase FRK
Patrick M Brauer et al. Cell Cycle. 2009.
Abstract
The Fyn related kinase FRK, originally called RAK, is a member of a small family of intracellular Src-related tyrosine kinases that includes PTK6 and Srms. These kinases share a conserved gene structure that is distinct from that of the Src family. Expression of FRK and PTK6 was originally identified in melanoma, breast cancer cells and normal intestinal epithelium, and both FRK and PTK6 have been implicated in the regulation of epithelial cell differentiation and apoptosis. Recently FRK was reported to phosphorylate the tumor suppressor PTEN (phosphatase and tensin homolog deleted from chromosome 10), a negative regulator of phosphatidylinositol 3 kinase (PI3K) signaling and AKT activation. FRK-mediated tyrosine phosphorylation of PTEN suppressed its association with NEDD4-1, an E3 ubiquitin ligase that may target it for polyubiquitination and proteosomal degradation. As a positive regulator of PTEN, FRK suppresses AKT signaling and inhibits breast cancer cell tumorgenicity in xenograft models. Both FRK and the related tyrosine kinase PTK6 appear to have multiple context-dependent functions, including the ability to regulate AKT. Although PTK6 negatively regulates AKT signaling in normal tissues in vivo, it may enhance AKT signaling in breast cancer cells. In contrast, FRK, which is expressed in the normal mammary gland but lost in some breast tumors, has tumor suppressor functions in mammary gland cells.
Figures
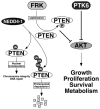
Figure 1
FRK and PTK6 negatively regulate AKT activity. The ubiquitin ligase NEDD4–1 can monoubiquinate or polyubiquitinate PTEN, leading to its nuclear translocation or proteosomal degradation respectively , . FRK directly phosphorylates PTEN and prevents its association with NEDD4–1, leading to stabilization and accumulation of PTEN . PTEN antagonizes the phosphoinositol-3-kinase/Akt pathway. PTK6 may directly associate with and phosphorylate AKT, and has been shown to inhibit AKT in unstimulated cells . Increased AKT activation has been detected in the intestines of Ptk6 null mice , .
Similar articles
- Functional interaction of phosphatase and tensin homologue (PTEN) with the E3 ligase NEDD4-1 during neuronal response to zinc.
Kwak YD, Wang B, Pan W, Xu H, Jiang X, Liao FF. Kwak YD, et al. J Biol Chem. 2010 Mar 26;285(13):9847-9857. doi: 10.1074/jbc.M109.091637. Epub 2010 Jan 25. J Biol Chem. 2010. PMID: 20100827 Free PMC article. - The tumour suppressor PTEN mediates a negative regulation of the E3 ubiquitin-protein ligase Nedd4.
Ahn Y, Hwang CY, Lee SR, Kwon KS, Lee C. Ahn Y, et al. Biochem J. 2008 Jun 1;412(2):331-8. doi: 10.1042/BJ20071403. Biochem J. 2008. PMID: 18307411 - Understanding the cellular roles of Fyn-related kinase (FRK): implications in cancer biology.
Goel RK, Lukong KE. Goel RK, et al. Cancer Metastasis Rev. 2016 Jun;35(2):179-99. doi: 10.1007/s10555-016-9623-3. Cancer Metastasis Rev. 2016. PMID: 27067725 Review. - Upregulation of Neural Precursor Cell Expressed Developmentally Downregulated 4-1 is Associated with Poor Prognosis and Chemoresistance in Lung Adenocarcinoma.
Song YH, Zhang CQ, Chen FF, Lin XY. Song YH, et al. Chin Med J (Engl). 2018 Jan 5;131(1):16-24. doi: 10.4103/0366-6999.221262. Chin Med J (Engl). 2018. PMID: 29271375 Free PMC article. - The FRK/RAK-SHB signaling cascade: a versatile signal-transduction pathway that regulates cell survival, differentiation and proliferation.
Annerén C, Lindholm CK, Kriz V, Welsh M. Annerén C, et al. Curr Mol Med. 2003 Jun;3(4):313-24. doi: 10.2174/1566524033479744. Curr Mol Med. 2003. PMID: 12776987 Review.
Cited by
- SH2 Domains Serve as Lipid-Binding Modules for pTyr-Signaling Proteins.
Park MJ, Sheng R, Silkov A, Jung DJ, Wang ZG, Xin Y, Kim H, Thiagarajan-Rosenkranz P, Song S, Yoon Y, Nam W, Kim I, Kim E, Lee DG, Chen Y, Singaram I, Wang L, Jang MH, Hwang CS, Honig B, Ryu S, Lorieau J, Kim YM, Cho W. Park MJ, et al. Mol Cell. 2016 Apr 7;62(1):7-20. doi: 10.1016/j.molcel.2016.01.027. Epub 2016 Mar 24. Mol Cell. 2016. PMID: 27052731 Free PMC article. - Regulation of PTEN stability and activity by Plk3.
Xu D, Yao Y, Jiang X, Lu L, Dai W. Xu D, et al. J Biol Chem. 2010 Dec 17;285(51):39935-42. doi: 10.1074/jbc.M110.166462. Epub 2010 Oct 12. J Biol Chem. 2010. PMID: 20940307 Free PMC article. - Involvement of Akt-1 and mTOR in sensitivity of breast cancer to targeted therapy.
Sokolosky ML, Stadelman KM, Chappell WH, Abrams SL, Martelli AM, Stivala F, Libra M, Nicoletti F, Drobot LB, Franklin RA, Steelman LS, McCubrey JA. Sokolosky ML, et al. Oncotarget. 2011 Jul;2(7):538-50. doi: 10.18632/oncotarget.302. Oncotarget. 2011. PMID: 21730367 Free PMC article. - Genome-Wide Selection Sweep Analysis to Identify Candidate Genes with Black and Brown Color in Tibetan Sibu Yaks.
Wu X, Xu L, Zhang H, Zhu Y, Zhang Q, Zhang C, E G. Wu X, et al. Animals (Basel). 2024 Aug 24;14(17):2458. doi: 10.3390/ani14172458. Animals (Basel). 2024. PMID: 39272243 Free PMC article. - Profiling Y561-dependent and -independent substrates of CSF-1R in epithelial cells.
Knowlton ML, Selfors LM, Wrobel CN, Gu TL, Ballif BA, Gygi SP, Polakiewicz R, Brugge JS. Knowlton ML, et al. PLoS One. 2010 Oct 26;5(10):e13587. doi: 10.1371/journal.pone.0013587. PLoS One. 2010. PMID: 21049007 Free PMC article.
References
- Pawson T, Kofler M. Kinome signaling through regulated protein-protein interactions in normal and cancer cells. Curr Opin Cell Biol. 2009;21:147–53. - PubMed
- Serfas MS, Tyner AL. Brk, Srm, Frk, and Src42A form a distinct family of intracellular Src-like tyrosine kinases. Oncol Res. 2003;13:409–19. - PubMed
- D’Aniello S, Irimia M, Maeso I, Pascual-Anaya J, Jimenez-Delgado S, Bertrand S, Garcia-Fernandez J. Gene expansion and retention leads to a diverse tyrosine kinase superfamily in amphioxus. Molecular biology and evolution. 2008;25:1841–54. - PubMed
- Thuveson M, Albrecht D, Zurcher G, Andres AC, Ziemiecki A. iyk, a novel intracellular protein tyrosine kinase differentially expressed in the mouse mammary gland and intestine [published erratum appears in Biochem Biophys Res Commun 1995 Jun 26;211(3):1100] Biochem Biophys Res Commun. 1995;209:582–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK068503/DK/NIDDK NIH HHS/United States
- R01 DK044525-15/DK/NIDDK NIH HHS/United States
- R01 DK044525/DK/NIDDK NIH HHS/United States
- DK068503/DK/NIDDK NIH HHS/United States
- DK44525/DK/NIDDK NIH HHS/United States
- R01 DK068503-04/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous