Expression and regulation of the NALP3 inflammasome complex in periodontal diseases - PubMed (original) (raw)
Expression and regulation of the NALP3 inflammasome complex in periodontal diseases
N Bostanci et al. Clin Exp Immunol. 2009 Sep.
Abstract
Periodontitis is an infectious process characterized by inflammation affecting the supporting structures of the teeth. Porphyromonas gingivalis is a major oral bacterial species implicated in the pathogenesis of periodontitis. Processing of interleukin (IL)-1 family cytokines is regulated by an intracellular innate immune response system, known as the NALP3 [nacht domain-, leucine-rich repeat-, and pyrin domain (PYD)-containing protein 3] inflammasome complex. The aim of the present study was to investigate by quantitative real-time polymerase chain reaction (PCR) the mRNA expression of NALP3, its effector molecule apoptosis associated speck-like protein (ASC), its putative antagonist NLRP2 (NLR family, PYD-containing protein 2), IL-1beta and IL-18 (i) in gingival tissues from patients with gingivitis (n = 10), chronic periodontitis (n = 18), generalized aggressive periodontitis (n = 20), as well as in healthy subjects (n = 20), (ii) in vitro in a human monocytic cell line (Mono-Mac-6), in response to P. gingivalis challenge for 6 h. The clinical data indicate that NALP3 and NLRP2, but not ASC, are expressed at significantly higher levels in the three forms of inflammatory periodontal disease compared to health. Furthermore, a positive correlation was revealed between NALP3 and IL-1beta or IL-18 expression levels in these tissues. The in vitro data demonstrate that P. gingivalis deregulates the NALP3 inflammasome complex in Mono-Mac-6 cells by enhancing NALP3 and down-regulating NLRP2 and ASC expression. In conclusion, this study reveals a role for the NALP3 inflammasome complex in inflammatory periodontal disease, and provides a mechanistic insight to the host immune responses involved in the pathogenesis of the disease by demonstrating the modulation of this cytokine-signalling pathway by bacterial challenge.
Figures
Fig. 1
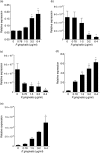
NALP3 [nacht domain-, leucine-rich repeat-, and pyrin domain (PYD)-containing protein 3] inflammasome complex expression levels in periodontal diseases. Distribution of NALP3 (a), NLRP2 (NLR family, PYD-containing protein 2) (b), ASC (c), interleukin (IL)-1β (d) and IL-18 (e) gene expression in gingival tissues of healthy subjects (n = 10) and patients with gingivitis (n = 10), chronic periodontitis (CP) (n = 18) and generalized aggressive periodontitis (G-AgP) (n = 20). The mRNA expression levels were measured by quantitative polymerase chain reaction (qPCR) analysis, normalized against the expression levels of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) and 18S RNA.
Fig. 2
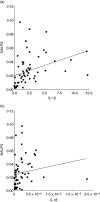
Scatter-plot showing the correlation between the NALP3 [nacht domain-, leucine-rich repeat-, and pyrin domain (PYD)-containing protein 3] and interleukin (IL)-1β (a) or IL-18 (b) mRNA expression in gingival tissues from all subjects (n = 58).
Fig. 3
Regulation of NALP3 [nacht domain-, leucine-rich repeat-, and pyrin domain (PYD)-containing protein 3] inflammasome complex expression by Porpyromonas gingivalis. Mono-Mac-6 cell cultures were challenged with ascending protein concentrations of P. gingivalis culture supernatant for 6 h. The mRNA expression levels of NALP-3 (a), NLRP2 (NLR family, PYD-containing protein 2) (b), ASC (c), interleukin (IL)-1β (d) and IL-18 (e) were measured by quantitative polymerase chain reaction (qPCR) analysis and normalized against the expression levels of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) and ubiquitin C (UBC). Bars represent mean values ± standard error of the mean from three independent experiments. The asterisk indicates the groups that were significantly different to the control group (P < 0·05).
Similar articles
- ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis.
Yilmaz O, Sater AA, Yao L, Koutouzis T, Pettengill M, Ojcius DM. Yilmaz O, et al. Cell Microbiol. 2010 Feb;12(2):188-98. doi: 10.1111/j.1462-5822.2009.01390.x. Epub 2009 Oct 6. Cell Microbiol. 2010. PMID: 19811501 Free PMC article. - [Study on the role of NALP3 inflammasome in Porphyromonas gingivalis lipopolysaccharide induced RAW264.7].
Yang F, Chen MY, Hu YY, Wang CN. Yang F, et al. Zhonghua Kou Qiang Yi Xue Za Zhi. 2017 May 9;52(5):289-293. doi: 10.3760/cma.j.issn.1002-0098.2017.05.006. Zhonghua Kou Qiang Yi Xue Za Zhi. 2017. PMID: 28482444 Chinese. - The Relationship between NALP3 and Autoinflammatory Syndromes.
Campbell L, Raheem I, Malemud CJ, Askari AD. Campbell L, et al. Int J Mol Sci. 2016 May 13;17(5):725. doi: 10.3390/ijms17050725. Int J Mol Sci. 2016. PMID: 27187378 Free PMC article. Review. - Historical aspects of studies on roles of the inflammasome in the pathogenesis of periodontal diseases.
Shibata K. Shibata K. Mol Oral Microbiol. 2018 Jun;33(3):203-211. doi: 10.1111/omi.12217. Epub 2018 Feb 20. Mol Oral Microbiol. 2018. PMID: 29360244 Review.
Cited by
- Porphyromonas gingivalis exacerbates the progression of fatty liver disease via CD36-PPARγ pathway.
Ahn JS, Yang JW, Oh SJ, Shin YY, Kang MJ, Park HR, Seo Y, Kim HS. Ahn JS, et al. BMB Rep. 2021 Jun;54(6):323-328. doi: 10.5483/BMBRep.2021.54.6.050. BMB Rep. 2021. PMID: 34078528 Free PMC article. - Relevance of Caspase-1 and Nlrp3 Inflammasome on Inflammatory Bone Resorption in A Murine Model of Periodontitis.
Rocha FRG, Delitto AE, de Souza JAC, González-Maldonado LA, Wallet SM, Rossa Junior C. Rocha FRG, et al. Sci Rep. 2020 May 8;10(1):7823. doi: 10.1038/s41598-020-64685-y. Sci Rep. 2020. PMID: 32385413 Free PMC article. - Chrysophanol inhibits NALP3 inflammasome activation and ameliorates cerebral ischemia/reperfusion in mice.
Zhang N, Zhang X, Liu X, Wang H, Xue J, Yu J, Kang N, Wang X. Zhang N, et al. Mediators Inflamm. 2014;2014:370530. doi: 10.1155/2014/370530. Epub 2014 Apr 29. Mediators Inflamm. 2014. PMID: 24876671 Free PMC article. - Porphyromonas gingivalis fimbriae dampen P2X7-dependent interleukin-1β secretion.
Morandini AC, Ramos-Junior ES, Potempa J, Nguyen KA, Oliveira AC, Bellio M, Ojcius DM, Scharfstein J, Coutinho-Silva R. Morandini AC, et al. J Innate Immun. 2014;6(6):831-45. doi: 10.1159/000363338. Epub 2014 Jun 6. J Innate Immun. 2014. PMID: 24925032 Free PMC article. - Titanium ions form particles that activate and execute interleukin-1β release from lipopolysaccharide-primed macrophages.
Pettersson M, Kelk P, Belibasakis GN, Bylund D, Molin Thorén M, Johansson A. Pettersson M, et al. J Periodontal Res. 2017 Feb;52(1):21-32. doi: 10.1111/jre.12364. Epub 2016 Mar 14. J Periodontal Res. 2017. PMID: 26987886 Free PMC article.
References
- Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. - PubMed
- Gemmell E, Marshall RI, Seymour GJ. Cytokines and prostaglandins in immune homeostasis and tissue destruction in periodontal disease. Periodontol 2000. 1997;14:112–43. - PubMed
- Taylor JJ, Preshaw PM, Donaldson PT. Cytokine gene polymorphism and immunoregulation in periodontal disease. Periodontol 2000. 2004;35:158–82. - PubMed
- Landi L, Amar S, Polins AS, Van Dyke TE. Host mechanisms in the pathogenesis of periodontal disease. Curr Opin Periodontol. 1997;4:3–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous