Epigenetic changes during disease progression in a murine model of human chronic lymphocytic leukemia - PubMed (original) (raw)
. 2009 Aug 11;106(32):13433-8.
doi: 10.1073/pnas.0906455106. Epub 2009 Jul 28.
Aparna Raval, Amy J Johnson, Erin Hertlein, Te-Hui Liu, Victor X Jin, Mara H Sherman, Shu-Jun Liu, David W Dawson, Katie E Williams, Mark Lanasa, Sandya Liyanarachchi, Thomas S Lin, Guido Marcucci, Yuri Pekarsky, Ramana Davuluri, Carlo M Croce, Denis C Guttridge, Michael A Teitell, John C Byrd, Christoph Plass
Affiliations
- PMID: 19666576
- PMCID: PMC2726368
- DOI: 10.1073/pnas.0906455106
Epigenetic changes during disease progression in a murine model of human chronic lymphocytic leukemia
Shih-Shih Chen et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Epigenetic alterations, including gain or loss of DNA methylation, are a hallmark of nearly every malignancy. Changes in DNA methylation can impact expression of cancer-related genes including apoptosis regulators and tumor suppressors. Because such epigenetic changes are reversible, they are being aggressively investigated as potential therapeutic targets. Here we use the Emu-TCL1 transgenic mouse model of chronic lymphocytic leukemia (CLL) to determine the timing and patterns of aberrant DNA methylation, and to investigate the mechanisms that lead to aberrant DNA methylation. We show that CLL cells from Emu-TCL1 mice at various stages recapitulate epigenetic alterations seen in human CLL. Aberrant methylation of promoter sequences is observed as early as 3 months of age in these animals, well before disease onset. Abnormally methylated promoter regions include binding sites for the transcription factor FOXD3. We show that loss of Foxd3 expression due to an NF-kappaB p50/p50:HDAC1 repressor complex occurs in TCL1-positive B cells before methylation. Therefore, specific transcriptional repression is an early event leading to epigenetic silencing of target genes in murine and human CLL. These results provide strong rationale for the development of strategies to target NF-kappaB components in CLL and potentially other B-cell malignancies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
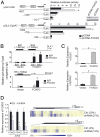
Fig. 1.
Specific DNA methylation profiling in Eμ-TCL1 mice recapitulates the changes in CLL patients. (A) MassARRAY analysis on CD19+ B cells, peripheral blood lymphocytes (PBL), Raji, WaC3CD5, and CLL B-cell samples. Each row represents a gene and each column indicates a sample. The heat map presents the quantitative methylation data from 0% to 100%. (B) Quantitative PCR results on CD19+ B-cell samples (n = 4) and CLL patients (n = 30). The expression level in the normal CD19+ B-cell samples was defined as 1. Error bars, s.e.m. (P ≤ 0.028 by Student t test). (C) Hierarchical cluster analysis with 5 TCL1 CLL leukemia, 8 Myc T/NK cell leukemia and 8 IL15 T cell leukemia mice, using all of the spots with at least 1 methylation (1,491 spots) across samples.
Fig. 2.
Early and accumulating DNA methylation in Eμ-TCL1 mice. (A) Average overall CpG methylation in TCL1 mice at 3, 5, 7, and 9 months (n = 3) and mice with advanced CLL (n = 5) as compared to WT mice at 4, 8, and 11 months (n = 2). P values given were calculated by Wilcoxon test. The significance of increased DNA methylation frequency overtime was calculated by Jonckheere-Terpstra test (P = 4.701e-06). (B) MassARRAY results of splenic B cells from WT (n = 4) or TCL1 mice (n = 2 for each age). (C) Expression of early methylated Sox3 and Gpc6 genes in TCL1 mouse splenic B cells compared to 3-month-old WT mice by SYBR-green PCR (n = 3, *P < 0.01 by Student t test). (D) Progressive re-activation of GPC6 expression after 0.5 μM 5aza-dC treatment over 12 days in Raji B-lymphoblastoid cells. (E) Increased expression of GPC6 in WaC3CD5 CLL cells treated dose-escalating concentrations (μM) of 5aza-dC for 3 days. The expression level of GPC6 was plotted relative to the expression level in the day 0 or 0 μM 5aza-dC amplified samples, respectively. Error bars, s.d.
Fig. 3.
High number methylated genes are downstream targets of repressed FOXD3 in CLL. (A) ChIP assay on 10 putative FOXD3 targets using 1-month-old WT mouse B-cell samples (n = 3). The relative FOXD3 pull down was normalized to a control IgG pull down for each gene analyzed. The extent of FOXD3 binding to methylated versus unmethylated target genes is significantly different by Fisher's Exact test (P = 0.001). (B) Methylation (Top) and expression (Bottom) of Foxd3 measured by COBRA and SYBR-Green PCR was compared between CD19+ B cells from WT and Eμ-TCL1 mice (P < 0.05 by Student t test). (C) FOXD3 Expression in CLL patients. Top: TaqMan PCR results from 49 CLL samples compared with 4 normal CD19+ B-cell samples (P = 0.0002 by Wilcoxon test). The box plot margins in (B) and (C) are the interquartile range with a thick line indicating the median. Each dot represents data from a single sample. Bottom: Foxd3 Immunoblot of B cells from 3 unaffected individuals and 6 CLL patients. (D) SYBR-Green PCR of FOXD3 target genes in splenic B cells from 1-month-old WT and TCL1 mice (n = 3, P < 0.04 by Student t test). (E) ChIP assay on splenic B cells from 1-month-old WT and TCL1 mice (n = 3, P < 0.05 by Student t test). The relative FOXD3 pull down was normalized to a control IgG pull down for each gene analyzed. Error bars, s.d.
Fig. 4.
Silenced and methylated FOXD3 targets in the knockdown lymphoblastic leukemia cell lines (A) Western blot (Right) shows FOXD3 expression in pcDNA3 vector and FOXD3 transfected Raji B cells. Promoter region schematics show 2 putative FOXD3 binding sites (BS1 and BS2) within the promoters of Dlx1 and EphA7. A gray bar indicates the location of a CpG island. Relative luciferase activity was normalized to the pGL3-basic vector transfection control (P < 0.05). (B) ChIP assays for FOXD3 predicted binding sites (BS) in DLX1 and EPHA7 promoters in control-pcDNA (vector) or pcDNA-FOXD3 (FOXD3) transfected Raji cells after 0.5 μM 5aza-dC treatments for 3 days. Diagrams on the top indicate a represent pull-down DNA for the indicated binding sites. The relative pull down of FOXD3 was normalized by a control IgG pull down (P < 0.001). (C) SYBR-Green PCR of DLX1 and EPHA7 in control or FOXD3 transfected Raji cells after a 3-day treatment of 0.5 μM 5aza-dC. The relative expression of each gene was normalized to data from the first amplified sample (P < 0.001). (D) Downregulated (Left) and methylated (Right) of DLX1, and PHF2 in Jurkat T-cells transfected with _FOXD3_-specific shRNA (P < 0.001). Schematics show the 5′ region of DLX1 and PHF2 genes. The black bars show the location of the analyzed amplicons. The average methylation percentage of both DLX1 and PHF2 amplicons is indicated. Error bars, s.d. Statistic analysis was done by Student t test.
Fig. 5.
Repressed FOXD3 in NF-κB transfected _TCL1_-overexpressed PBL cell line. (A) Transformed PBL cells were transfected with a control vector (PBL-puro) or TCL1 (PBL-TCL1). FOXD3 expression was analyzed by SYBR-Green PCR (P = 0.007). (B) Luciferase assay (Top) for FOXD3 promoter activity using a FOXD3 reporter containing the putative NF-κB binding site (gray square, the sequence is shown in C; P = 0.005). (Bottom) Identification of a conserved, putative NF-κB binding site in the mouse and human Foxd3 promoters (underlined). (C) Luciferase assay for promoter activity using a FOXD3 reporter construct and 500 ng of each NF-κB subunit expression vector. Cell lysates were collected after 48 h. Relative promoter activity was normalized to pCMV transfected PBL-puro cells. Results are averaged from triplicate assays. Error bars, s.d. (P = 0.049); all P values were calculated by Student t test.
Fig. 6.
FOXD3 is repressed by NF-κB p50:HDAC1 complex in TCL1-expressing cells. (A) EMSA assays (Left) with the NF-κB binding sequence of the FOXD3 promoter and nuclear extract from normal (n = 3) and CLL patient B-cell samples (n = 6). Supershift assay (Right) using B-cell samples from an additional patient with antibodies against p65, p50, HDAC1 (HD1), HDAC3 (HD3), and IgG. (B) ChIP assay (Top) surveying the FOXD3 NF-κB promoter site in B cells from CLL patients and normal individuals. ChIP assay (Bottom) surveying the Foxd3 NF-κB promoter site in splenic B cells from WT and 1-month-old TCL1 mice. The data were normalized to 1.0 by IgG pull down (P < 0.05 by Student t test). Error bars in the figures indicate s.d. (C) Western blots on 30 μg nuclear extract (NE) or cytoplasm extract (CE) from CLL patient and normal B-cell samples. (D) Western blots on 30 μg nuclear extract (NE) or cytoplasm extract (CE) of sorted B cells from 3 pooled 1-month-old TCL1 or WT mice. The nuclear protein PARP was used as an internal control. (E) Nuclear extract (500 μg) per sample from a normal donor or pooled from 2 CLL patient's B cells were immunoprecipitated (IP) with TCL1 (Left), p50 (Right) or control antibody against PARP, followed by Western blot analysis (WB) with antibodies against p50 or TCL1.
Similar articles
- Epigenetic alterations in a murine model for chronic lymphocytic leukemia.
Chen SS, Sherman MH, Hertlein E, Johnson AJ, Teitell MA, Byrd JC, Plass C. Chen SS, et al. Cell Cycle. 2009 Nov 15;8(22):3663-7. doi: 10.4161/cc.8.22.9957. Cell Cycle. 2009. PMID: 19901553 Free PMC article. Review. - Epigenetic deregulation in chronic lymphocytic leukemia: Clinical and biological impact.
Mansouri L, Wierzbinska JA, Plass C, Rosenquist R. Mansouri L, et al. Semin Cancer Biol. 2018 Aug;51:1-11. doi: 10.1016/j.semcancer.2018.02.001. Epub 2018 Feb 7. Semin Cancer Biol. 2018. PMID: 29427646 Review. - Silencing of the inhibitor of DNA binding protein 4 (ID4) contributes to the pathogenesis of mouse and human CLL.
Chen SS, Claus R, Lucas DM, Yu L, Qian J, Ruppert AS, West DA, Williams KE, Johnson AJ, Sablitzky F, Plass C, Byrd JC. Chen SS, et al. Blood. 2011 Jan 20;117(3):862-71. doi: 10.1182/blood-2010-05-284638. Epub 2010 Nov 22. Blood. 2011. PMID: 21098398 Free PMC article. - NF-κB p50 (nfkb1) contributes to pathogenesis in the Eμ-TCL1 mouse model of chronic lymphocytic leukemia.
Chen TL, Tran M, Lakshmanan A, Harrington BK, Gupta N, Goettl VM, Lehman AM, Trudeau S, Lucas DM, Johnson AJ, Byrd JC, Hertlein E. Chen TL, et al. Blood. 2017 Jul 20;130(3):376-379. doi: 10.1182/blood-2017-01-761130. Epub 2017 May 17. Blood. 2017. PMID: 28515090 Free PMC article. No abstract available. - A Sleeping Beauty screen reveals NF-kB activation in CLL mouse model.
Zanesi N, Balatti V, Riordan J, Burch A, Rizzotto L, Palamarchuk A, Cascione L, Lagana A, Dupuy AJ, Croce CM, Pekarsky Y. Zanesi N, et al. Blood. 2013 May 23;121(21):4355-8. doi: 10.1182/blood-2013-02-486035. Epub 2013 Apr 16. Blood. 2013. PMID: 23591791 Free PMC article.
Cited by
- FOXD3 modulates migration through direct transcriptional repression of TWIST1 in melanoma.
Weiss MB, Abel EV, Dadpey N, Aplin AE. Weiss MB, et al. Mol Cancer Res. 2014 Sep;12(9):1314-23. doi: 10.1158/1541-7786.MCR-14-0170. Epub 2014 Jul 24. Mol Cancer Res. 2014. PMID: 25061102 Free PMC article. - Active DNA demethylation in human postmitotic cells correlates with activating histone modifications, but not transcription levels.
Klug M, Heinz S, Gebhard C, Schwarzfischer L, Krause SW, Andreesen R, Rehli M. Klug M, et al. Genome Biol. 2010;11(6):R63. doi: 10.1186/gb-2010-11-6-r63. Epub 2010 Jun 18. Genome Biol. 2010. PMID: 20565882 Free PMC article. - Chronic lymphocytic leukemia--genomics lead the way.
Mertens D, Bullinger L, Stilgenbauer S. Mertens D, et al. Haematologica. 2011 Oct;96(10):1402-5. doi: 10.3324/haematol.2011.052175. Haematologica. 2011. PMID: 21972210 Free PMC article. No abstract available. - Proteomics and metabolomics identify molecular mechanisms of aging potentially predisposing for chronic lymphocytic leukemia.
Mayer RL, Schwarzmeier JD, Gerner MC, Bileck A, Mader JC, Meier-Menches SM, Gerner SM, Schmetterer KG, Pukrop T, Reichle A, Slany A, Gerner C. Mayer RL, et al. Mol Cell Proteomics. 2018 Feb;17(2):290-303. doi: 10.1074/mcp.RA117.000425. Epub 2017 Dec 1. Mol Cell Proteomics. 2018. PMID: 29196338 Free PMC article. - Genome-wide DNA methylation analysis reveals novel epigenetic changes in chronic lymphocytic leukemia.
Pei L, Choi JH, Liu J, Lee EJ, McCarthy B, Wilson JM, Speir E, Awan F, Tae H, Arthur G, Schnabel JL, Taylor KH, Wang X, Xu D, Ding HF, Munn DH, Caldwell C, Shi H. Pei L, et al. Epigenetics. 2012 Jun 1;7(6):567-78. doi: 10.4161/epi.20237. Epub 2012 Jun 1. Epigenetics. 2012. PMID: 22534504 Free PMC article.
References
- Weber M, et al. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet. 2005;37:853–862. - PubMed
- Suganuma T, Workman JL. Crosstalk among histone modifications. Cell. 2008;135:604–607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA81534/CA/NCI NIH HHS/United States
- A101956/PHS HHS/United States
- P30 CA16058/CA/NCI NIH HHS/United States
- P01 CA081534/CA/NCI NIH HHS/United States
- T32 CA106196/CA/NCI NIH HHS/United States
- R21 CA110496/CA/NCI NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
- CA110496/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous