Linking the p53 tumour suppressor pathway to somatic cell reprogramming - PubMed (original) (raw)
. 2009 Aug 27;460(7259):1140-4.
doi: 10.1038/nature08311. Epub 2009 Aug 9.
Affiliations
- PMID: 19668186
- PMCID: PMC2735889
- DOI: 10.1038/nature08311
Linking the p53 tumour suppressor pathway to somatic cell reprogramming
Teruhisa Kawamura et al. Nature. 2009.
Abstract
Reprogramming somatic cells to induced pluripotent stem (iPS) cells has been accomplished by expressing pluripotency factors and oncogenes, but the low frequency and tendency to induce malignant transformation compromise the clinical utility of this powerful approach. We address both issues by investigating the mechanisms limiting reprogramming efficiency in somatic cells. Here we show that reprogramming factors can activate the p53 (also known as Trp53 in mice, TP53 in humans) pathway. Reducing signalling to p53 by expressing a mutated version of one of its negative regulators, by deleting or knocking down p53 or its target gene, p21 (also known as Cdkn1a), or by antagonizing reprogramming-induced apoptosis in mouse fibroblasts increases reprogramming efficiency. Notably, decreasing p53 protein levels enabled fibroblasts to give rise to iPS cells capable of generating germline-transmitting chimaeric mice using only Oct4 (also known as Pou5f1) and Sox2. Furthermore, silencing of p53 significantly increased the reprogramming efficiency of human somatic cells. These results provide insights into reprogramming mechanisms and suggest new routes to more efficient reprogramming while minimizing the use of oncogenes.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures
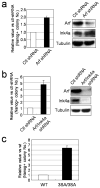
Fig. 1. Increased generation of iPS cells by blocking p53 and p21
(a) MEFs were infected by retroviruses encoding 3 factors (Oct4/Sox2/Klf4), 2 factors (Oct4/Sox2), Klf4, c-Myc or GFP. Four days after infection, the protein levels of p53, Arf, and p21 were analyzed by Western Blotting. α-Tubulin was used as a loading control. (b) MEFs were infected by 3F (Oct4/Sox2/Klf4) in combination with mock, control shRNA (GFP) and p53 shRNA (#1 and #2). Emerging colonies of iPS cells were visualized by immunostaining with anti-Nanog antibody using an Avidin Biotin Complex (ABC) method. (c) The fold change in the number of Nanog-positive colonies compared to mock (n=4). For all figures in this study, error bars indicate s.d. p53 knockdown efficiency was examined by western blot. (d) MEFs were infected by 3F in combination with mock, p53 shRNA and p21 shRNA. Four days later, half of cells were treated with Nutlin 3a (0, 3, 10μM) and analyzed for p53 level. The remainder were stained for Nanog-positive colonies. (e) Immunostaining of Nanog positive colonies generated from p53(+/+), p53(+/−) and p53(−/−) MEFs by 3F showed p53 dose-dependent decrease of colony number. (f) Retroviral infection of p53 into p53(−/−) MEF decreased the number of Nanog-positive colonies induced by 3F. p53 and p21 levels on day 3 after infection were analyzed. (g) Nutlin 3a dramatically reduced reprogramming of p53(+/+) MEFs, but not on p53(−/−) MEF. (h) Fold change in the number of Nanog-positive colonies by p21 shRNA (n=4). p21 knockdown efficiency was examined by western blot.
Fig. 2. Modulation of p53 activity alters reprogramming efficiency
(a) and (b) Fold change in the number of 3F induced Nanog-positive colonies by Arf shRNA or by Arf/Ink4a shRNA compared to control shRNA (n=3). Protein knockdown efficiency was examined by western blot. (c) 3F induced Nanog-positive colonies from wild type (+/+) and homozygous (3SA/3SA) MEFs (n=3).
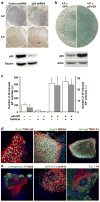
Fig. 3. Generation and characterization of 2F-p53KD-iPS cells by p53 downregulation
(a) Morphology and GFP fluorescence of 2F-p53KD-iPS cell lines. GFP expression is silenced in clone #6. (b) Alkaline phosphatase staining of 2F-p53KD-iPS cell lines. DAPI was used to visualize cell nuclei. (c) Protein levels of Nanog, Oct4, Sox2, Klf4, c-Myc, p53 in 2F-p53KD-iPS cell lines are shown. α-Tubulin was used as loading control. (d) Embryoid bodies (EBs) of 2F-p53KD-iPS cell clones on day 6 of differentiation. EBs were transferred to gelatinized dishes on day 3 to 5 for further differentiation. On day 14, EBs were subjected to immunofluorescence for α-fetoprotein (AFP)/Foxa2 (endoderm), α-sarcomeric actin/GATA4 (mesoderm) and Tuj1/GFAP (ectoderm). (e) Immunofluorescence of teratoma from 2F-p53KD-iPS cells by antibodies against AFP/Foxa2 (endoderm), α-sarcomeric actinin/Chondroitin (mesoderm), Tuj1/GFAP (ectoderm) showed spontaneous differentiation into all three germ layers. (f) Adult chimeric mice obtained from 2F-p53KD iPS lines (#1 and #6) and non-chimeric mouse in C57BL/6J host blastocysts. (g) As of the date of submission, the mating of offspring from clone #6 chimera to a C57BL/6J female generated 1 agouti pup (blue arrow), that together with PCR analysis (not shown) indicate germ line transmission of the 2F-iPS genome.
Fig. 4. Downregulation of p53 activity increases reprogramming efficiency of human somatic cells
(a) Human embryonic fibroblasts were infected with retroviruses encoding Oct4/Sox2/Klf4 (3-F) or Oct4/Sox2/Klf4/c-Myc (4-F) factors in combination with lentiviruses expressing control-or p53-shRNA. Emerging colonies of iPS cells were immunostained with anti-Nanog antibody. p53 knockdown efficiency was examined by western blot. (b) Human primary keratinocytes were co-infected with 4-F and retroviruses expressing GFP or p53-DD. Two weeks later, cells were stained for AP activity. Expression of p53-DD resulted in stabilization of wild-type p53 (lower panels). Actin was used as a loading control. (c) The bars represent the average number of iPS-like colonies obtained from 10 keratinocytes reprogrammed with 3F or 4F and retroviruses encoding GFP or p53-DD, in the absence or presence of Nutlin3a (n=3). iPS-like colonies were scored as having hES-like morphology and positive AP staining. Due to the numerous colonies generated in 4F p53-DD keratinocytes, quantification was done using 10 cells. (d, e) Colonies of human keratinocyte-derived iPS cells generated by 3F and p53-DD display strong immunoreactivity for pluripotency-associated transcription factors and surface markers (d) and differentiate in vitro into cell types that express markers of endoderm (α-fetoprotein, FoxA2), mesoderm (GATA4, sarcomeric α-actinin), and ectoderm (Tuj1, TH) (e).
Comment in
- Stem cells: The promises and perils of p53.
Krizhanovsky V, Lowe SW. Krizhanovsky V, et al. Nature. 2009 Aug 27;460(7259):1085-6. doi: 10.1038/4601085a. Nature. 2009. PMID: 19713919 Free PMC article.
Similar articles
- Suppression of induced pluripotent stem cell generation by the p53-p21 pathway.
Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Hong H, et al. Nature. 2009 Aug 27;460(7259):1132-5. doi: 10.1038/nature08235. Epub 2009 Aug 9. Nature. 2009. PMID: 19668191 Free PMC article. - Immortalization eliminates a roadblock during cellular reprogramming into iPS cells.
Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM, Khalil A, Rheinwald JG, Hochedlinger K. Utikal J, et al. Nature. 2009 Aug 27;460(7259):1145-8. doi: 10.1038/nature08285. Epub 2009 Aug 9. Nature. 2009. PMID: 19668190 Free PMC article. - The Ink4/Arf locus is a barrier for iPS cell reprogramming.
Li H, Collado M, Villasante A, Strati K, Ortega S, Cañamero M, Blasco MA, Serrano M. Li H, et al. Nature. 2009 Aug 27;460(7259):1136-9. doi: 10.1038/nature08290. Epub 2009 Aug 9. Nature. 2009. PMID: 19668188 Free PMC article. - SOX2 and p53 Expression Control Converges in PI3K/AKT Signaling with Versatile Implications for Stemness and Cancer.
Schaefer T, Steiner R, Lengerke C. Schaefer T, et al. Int J Mol Sci. 2020 Jul 11;21(14):4902. doi: 10.3390/ijms21144902. Int J Mol Sci. 2020. PMID: 32664542 Free PMC article. Review. - Advances in reprogramming somatic cells to induced pluripotent stem cells.
Patel M, Yang S. Patel M, et al. Stem Cell Rev Rep. 2010 Sep;6(3):367-80. doi: 10.1007/s12015-010-9123-8. Stem Cell Rev Rep. 2010. PMID: 20336395 Free PMC article. Review.
Cited by
- The cell cycle and pluripotency.
Hindley C, Philpott A. Hindley C, et al. Biochem J. 2013 Apr 15;451(2):135-43. doi: 10.1042/BJ20121627. Biochem J. 2013. PMID: 23535166 Free PMC article. Review. - NF-κB activation impairs somatic cell reprogramming in ageing.
Soria-Valles C, Osorio FG, Gutiérrez-Fernández A, De Los Angeles A, Bueno C, Menéndez P, Martín-Subero JI, Daley GQ, Freije JM, López-Otín C. Soria-Valles C, et al. Nat Cell Biol. 2015 Aug;17(8):1004-13. doi: 10.1038/ncb3207. Epub 2015 Jul 27. Nat Cell Biol. 2015. PMID: 26214134 Retracted. - Mechanisms of Congenital Malformations in Pregnancies with Pre-existing Diabetes.
Loeken MR. Loeken MR. Curr Diab Rep. 2020 Sep 12;20(10):54. doi: 10.1007/s11892-020-01338-4. Curr Diab Rep. 2020. PMID: 32918152 Free PMC article. Review. - Reprogramming of gastrointestinal cancer cells.
Dewi D, Ishii H, Haraguchi N, Nishikawa S, Kano Y, Fukusumi T, Ohta K, Miyazaki S, Ozaki M, Sakai D, Satoh T, Nagano H, Doki Y, Mori M. Dewi D, et al. Cancer Sci. 2012 Mar;103(3):393-9. doi: 10.1111/j.1349-7006.2011.02184.x. Epub 2012 Jan 17. Cancer Sci. 2012. PMID: 22151786 Free PMC article. Review. - p53 counteracts reprogramming by inhibiting mesenchymal-to-epithelial transition.
Brosh R, Assia-Alroy Y, Molchadsky A, Bornstein C, Dekel E, Madar S, Shetzer Y, Rivlin N, Goldfinger N, Sarig R, Rotter V. Brosh R, et al. Cell Death Differ. 2013 Feb;20(2):312-20. doi: 10.1038/cdd.2012.125. Epub 2012 Sep 21. Cell Death Differ. 2013. PMID: 22996684 Free PMC article.
References
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. - PubMed
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. - PubMed
- Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. - PubMed
- Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. - PubMed
Additional References
- Kitamura T, et al. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol. 2003;31:1007–14. - PubMed
- Sherley JL. Guanine nucleotide biosynthesis is regulated by the cellular p53 concentration. J Biol Chem. 1991;266:24815–28. - PubMed
- Huppi K, et al. Molecular cloning, sequencing, chromosomal localization and expression of mouse p21 (Waf1) Oncogene. 1994;9:3017–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5 R01 CA100845/CA/NCI NIH HHS/United States
- R01 CA100845/CA/NCI NIH HHS/United States
- R33 HL088293/HL/NHLBI NIH HHS/United States
- 5 R01 CA061449/CA/NCI NIH HHS/United States
- R01 CA061449/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous