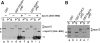Syp1 is a conserved endocytic adaptor that contains domains involved in cargo selection and membrane tubulation - PubMed (original) (raw)
Syp1 is a conserved endocytic adaptor that contains domains involved in cargo selection and membrane tubulation
Amanda Reider et al. EMBO J. 2009.
Abstract
Internalization of diverse transmembrane cargos from the plasma membrane requires a similarly diverse array of specialized adaptors, yet only a few adaptors have been characterized. We report the identification of the muniscin family of endocytic adaptors that is conserved from yeast to human beings. Solving the structures of yeast muniscin domains confirmed the unique combination of an N-terminal domain homologous to the crescent-shaped membrane-tubulating EFC/F-BAR domains and a C-terminal domain homologous to cargo-binding mu homology domains (muHDs). In vitro and in vivo assays confirmed membrane-tubulation activity for muniscin EFC/F-BAR domains. The muHD domain has conserved interactions with the endocytic adaptor/scaffold Ede1/eps15, which influences muniscin localization. The transmembrane protein Mid2, earlier implicated in polarized Rho1 signalling, was identified as a cargo of the yeast adaptor protein. These and other data suggest a model in which the muniscins provide a combined adaptor/membrane-tubulation activity that is important for regulating endocytosis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
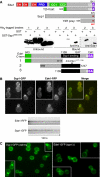
Syp1 and Ede1 interact directly and colocalize in vivo. (A) Upper: schematic of fragments used in the yeast two-hybrid (Y2H) experiment. EH, EH domain; PRD, proline-rich domain; CC, coiled coil; UBA, ubiquitin-associated domain; NPF, Asn-Pro-Phe tripeptide. Lower: recombinant protein-binding assay with GST or GST-Syp1566−870 bound to glutathione agarose beads incubated with lysates from bacteria expressing His6-tagged fragment of Ede1 as indicated in the schematic, and immunblotted with anti-His6 or anti-Ede11177−1190 antibodies. The black bar encompassing Ede11109−1247 constitutes the minimal-binding domain for Syp1566−870. (B) Upper: colocalization of Ede1-RFP and Syp1-GFP, each expressed from the endogenous gene with an integrated C-terminal fluorescent fusion protein. Lower: kymographs of time-lapse movies showing colocalization and similar dynamics of Ede1-RFP and Syp1-GFP proteins at cortical patches. (C) Localization of Syp1-GFP in _ede1_Δ cells and Ede1-GFP in _syp1_Δ cells. Images taken by fluorescence microscopy with appropriate filter sets. A full-colour version of this figure is available at The EMBO Journal Online.
Figure 2
Syp1 is an endocytic protein. (A) Complementation of LY uptake defect in _ede1_Δ+pRS416 (vector) cells by CEN EDE1 (single copy) and partial suppression by 2μSYP1 (high copy). Cells were incubated with 4 mg/ml LY for 1.5 h at 30°C, washed, and examined by fluorescence microscopy with FITC filters. (B) Quantification of LY uptake experiment. For each condition, cells were quantified as strong (+), intermediate (±), or weak/absent (−) vacuolar signal, and the percentage in each category was plotted. _ede1_Δ+vector, _n_=107 cells; _ede1_Δ+CEN EDE1, _n_=94 cells; _ede1_Δ+2_μ_SYP1, _n_=88 cells; * represents statistical analysis using Fisher's exact test, two-tailed ‘mid-P' calculation <0.05.
Figure 3
Syp1 has EFC/F-BAR and μHD domains. (A) Human homologues of Syp1; % identity between the Syp1 domain and the human domains is indicated. EFC/F-BAR, extended FCH/FCH-BAR domain; PRD, proline-rich domain; μHD, μ-subunit homology domain; green box, region of homology shared by human proteins. (B) Structure of the Syp1 EFC/F-BAR domain dimer. The dimeric module is composed of two monomers (coloured in green and light green) related by two-fold crystallographic symmetry axis in the crystal. The basic Lys residues selected for mutagenesis on the membrane-binding surface are shown in stick models. The pink solid line shows a putative curvature of the membrane-binding surface of Syp1. (C) Alignment of the core helical bundles of the EFC/F-BAR domains of Syp1 (green) and FCHO2 (magenta). For the alignment of the central helix bundles, 75 Cα atoms from each monomer were used for superposition (see Materials and methods). (D) Structural model for the Syp1 μHD; the NPF (Asn-Pro-Phe) tripeptide motif is shown in stick models on the right side. (E) Superposition of the μHD domains of Syp1 (green) and μ2 subunit of AP-2 (magenta); 154 equivalent residues out of 262 residues in Syp1 μHD were selected for superposition, resulting in an r.m.s.d. of 1.58 Å.
Figure 4
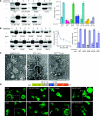
Syp1 domains mediate membrane binding in vitro and in vivo. (A) Full-length Syp1 and the N-, middle, and C-terminal domains of Syp1 were tested for binding to liposomes. Upper: Folch liposomes (100 nm diameter) were mixed with Syp11−870 (full-length), Syp11−265 (EFC/F-BAR), Syp1265−565 (middle), Syp1566−870 (μHD), pelleted, and supernatant versus pellet fractions separated by SDS–PAGE and stained with Coomassie. An identical experiment without liposomes (−) is shown. Lower: same experiment as above, but mapping the liposome-binding site in the middle fragment with Syp1265−365, Syp1366−465, and Syp1466−565. Quantification of three independent experiments is in the graph to the right; average±s.d. are shown. *unpaired _t_-test _P_-value=0.002. (B) Ionic interactions contribute to liposome binding by the Syp1 EFC/F-BAR domain. Upper: increasing [imidazole] was mixed with Syp11−265, then tested for binding to liposomes. 10, 20, 40, 80, and 160 mM imidazole-containing buffer was used. Lower: Syp11−265 WT, K37E, K44E, K131E, and K37E/K44E/K131E triple mutant (3K-E) protein was tested for liposome binding as above using 10 mM imidazole-containing buffer. Quantification is in graphs to the right; average±s.d. are shown. (C) Tubulation of Folch liposomes was examined by negative stain EM; 100 nm liposomes were mixed with recombinant Syp1 middle or EFC/F-BAR domain (WT or 3K-E) protein for 1 or 5 min, adsorbed to a carbon-coated grid and stained with uranyl acetate. The average tubule diameter for the WT EFC domain was measured to be 18.1±2.2 nm (_n_=91). Scale bar, 200 nm. (D) Requirement of Syp1 domains for proper localization in vivo was tested with truncated proteins fused to GFP at the C-termini. Arrowheads indicate punctate or highly polarized localization of a GFP chimeric protein. Magnified views of Syp11−870-GFP, Syp11−565-GFP, and Syp1566−870-GFP are shown in the insets, noted as (+). The schematic indicates the positions of each truncation, asterisks denote the lysine-rich putative PtdIns(4,5)P2-binding sites. A full-colour version of this figure is available at The EMBO Journal Online.
Figure 5
μHDs of FCHO1 and Syp1 bind eps15. (A) Approximately 200 μg of GST (lanes a, b), GST-FCHO1 μHD (lanes c–f) or GST-SGIP1 μHD (lanes g–j) immobilized on beads was incubated with clarified rat brain cytosol in the absence or presence (lanes e, f, i, j) of 25 μM eps15 (594–896) polypeptide as indicated. Aliquots of each supernatant (S) and washed pellet (P) were resolved by SDS–PAGE, transferred to nitrocellulose, and probed with anti-eps15 antibodies; only the relevant portions are shown. The eps15 competitor peptide and a degradation product (open arrowheads) are shown. Two non-specific bands detected by the antibody are indicated (asterisks). (B) Approximately 200 μg of GST (lanes a, b) GST-FCHO1 μHD (lanes c–d) or GST-Syp1 μHD (lanes e–f) immobilized on beads was incubated with clarified rat brain cytosol and analysed similarly.
Figure 6
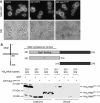
Mid2 is a Syp1 cargo. (A) Localization of Mid2-GFP in WT cells expressing high copy empty vector, SYP1 or syp1_Δμ_HD was examined by fluorescence microscopy. (B) Lysates with recombinant His6-Mid2 cytosolic tail (aa 251–376, aa 251–316, aa 317–376) were incubated with bead-immobilized GST or GST-Syp1 μHD, washed, and lysates versus bound fractions separated on 12–20% SDS–PAGE and immunoblotted with anti-His6 antibodies.
Figure 7
FCHO1 localization and function indicates that it is an endocytic protein. Top row and bottom left: representative images of fixed HeLa cells transiently transfected with GFP-FCHO1, stained for clathrin, AP-2 (α subunit), and eps15 as indicated. Bottom right: transiently transfected cells were incubated on ice with anti-LDL receptor mAb IgG-C7 for 60 min before warming to 37°C as indicated. The accumulation of LDL receptors over the PM and in a diffuse form in GFP-FCHO1-expressing cells can be seen. Scale bar, 10 μm.
Similar articles
- The conserved protein adaptors CALM/AP180 and FCHo1/2 cooperatively recruit Eps15 to promote the initiation of clathrin-mediated endocytosis in yeast.
Sun Y, Yeam A, Kuo J, Iwamoto Y, Hu G, Drubin DG. Sun Y, et al. PLoS Biol. 2024 Sep 24;22(9):e3002833. doi: 10.1371/journal.pbio.3002833. eCollection 2024 Sep. PLoS Biol. 2024. PMID: 39316607 Free PMC article. - Syp1 regulates the clathrin-mediated and clathrin-independent endocytosis of multiple cargo proteins through a novel sorting motif.
Apel AR, Hoban K, Chuartzman S, Tonikian R, Sidhu S, Schuldiner M, Wendland B, Prosser D. Apel AR, et al. Mol Biol Cell. 2017 Sep 1;28(18):2434-2448. doi: 10.1091/mbc.E15-10-0731. Epub 2017 Jul 12. Mol Biol Cell. 2017. PMID: 28701344 Free PMC article. - Yeast endocytic adaptor AP-2 binds the stress sensor Mid2 and functions in polarized cell responses.
Chapa-y-Lazo B, Allwood EG, Smaczynska-de Rooij II, Snape ML, Ayscough KR. Chapa-y-Lazo B, et al. Traffic. 2014 May;15(5):546-57. doi: 10.1111/tra.12155. Epub 2014 Feb 25. Traffic. 2014. PMID: 24460703 Free PMC article. - The early steps of endocytosis: from cargo selection to membrane deformation.
Rao Y, Rückert C, Saenger W, Haucke V. Rao Y, et al. Eur J Cell Biol. 2012 Apr;91(4):226-33. doi: 10.1016/j.ejcb.2011.02.004. Epub 2011 Mar 31. Eur J Cell Biol. 2012. PMID: 21458101 Review. - Ubiquitin ligase adaptors: regulators of ubiquitylation and endocytosis of plasma membrane proteins.
Léon S, Haguenauer-Tsapis R. Léon S, et al. Exp Cell Res. 2009 May 15;315(9):1574-83. doi: 10.1016/j.yexcr.2008.11.014. Epub 2008 Dec 3. Exp Cell Res. 2009. PMID: 19070615 Review.
Cited by
- The conserved protein adaptors CALM/AP180 and FCHo1/2 cooperatively recruit Eps15 to promote the initiation of clathrin-mediated endocytosis in yeast.
Sun Y, Yeam A, Kuo J, Iwamoto Y, Hu G, Drubin DG. Sun Y, et al. PLoS Biol. 2024 Sep 24;22(9):e3002833. doi: 10.1371/journal.pbio.3002833. eCollection 2024 Sep. PLoS Biol. 2024. PMID: 39316607 Free PMC article. - Quantification of cytosolic interactions identifies Ede1 oligomers as key organizers of endocytosis.
Boeke D, Trautmann S, Meurer M, Wachsmuth M, Godlee C, Knop M, Kaksonen M. Boeke D, et al. Mol Syst Biol. 2014 Nov 3;10(11):756. doi: 10.15252/msb.20145422. Mol Syst Biol. 2014. PMID: 25366307 Free PMC article. - Structural basis for the recognition of tyrosine-based sorting signals by the μ3A subunit of the AP-3 adaptor complex.
Mardones GA, Burgos PV, Lin Y, Kloer DP, Magadán JG, Hurley JH, Bonifacino JS. Mardones GA, et al. J Biol Chem. 2013 Mar 29;288(13):9563-71. doi: 10.1074/jbc.M113.450775. Epub 2013 Feb 12. J Biol Chem. 2013. PMID: 23404500 Free PMC article. - The EH domain-containing protein, EdeA, is involved in endocytosis, cell wall integrity, and pathogenicity in Aspergillus fumigatus.
Dai M, Liu X, Goldman GH, Lu L, Zhang S. Dai M, et al. mSphere. 2024 May 29;9(5):e0005724. doi: 10.1128/msphere.00057-24. Epub 2024 Apr 30. mSphere. 2024. PMID: 38687129 Free PMC article. - Regulation of clathrin coat assembly by Eps15 homology domain-mediated interactions during endocytosis.
Suzuki R, Toshima JY, Toshima J. Suzuki R, et al. Mol Biol Cell. 2012 Feb;23(4):687-700. doi: 10.1091/mbc.E11-04-0380. Epub 2011 Dec 21. Mol Biol Cell. 2012. PMID: 22190739 Free PMC article.
References
- Aguilar RC, Watson HA, Wendland B (2003) The yeast Epsin Ent1 is recruited to membranes through multiple independent interactions. J Biol Chem 278: 10737–10743 - PubMed
- Baggett JJ, Shaw JD, Sciambi CJ, Watson HA, Wendland B (2003b) Fluorescent labeling of yeast. Curr Protoc Cell Biol Chapter 4: Unit 413 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM007231/GM/NIGMS NIH HHS/United States
- R01 DK53249/DK/NIDDK NIH HHS/United States
- R01 DK053249/DK/NIDDK NIH HHS/United States
- GM07231/GM/NIGMS NIH HHS/United States
- R01 GM060979/GM/NIGMS NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R01 GM60979/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous