Glucolipotoxicity of the pancreatic beta cell - PubMed (original) (raw)
Review
Glucolipotoxicity of the pancreatic beta cell
Vincent Poitout et al. Biochim Biophys Acta. 2010 Mar.
Abstract
The concept of glucolipotoxicity refers to the combined, deleterious effects of elevated glucose and fatty acid levels on pancreatic beta-cell function and survival. Significant progress has been made in recent years towards a better understanding of the cellular and molecular basis of glucolipotoxicity in the beta cell. The permissive effect of elevated glucose on the detrimental actions of fatty acids stems from the influence of glucose on intracellular fatty acid metabolism, promoting the synthesis of cellular lipids. The combination of excessive levels of fatty acids and glucose therefore leads to decreased insulin secretion, impaired insulin gene expression, and beta-cell death by apoptosis, all of which probably have distinct underlying mechanisms. Recent studies from our laboratory have identified several pathways implicated in fatty acid inhibition of insulin gene expression, including the extracellular-regulated kinase (ERK1/2) pathway, the metabolic sensor Per-Arnt-Sim kinase (PASK), and the ATF6 branch of the unfolded protein response. We have also confirmed in vivo in rats that the decrease in insulin gene expression is an early defect which precedes any detectable abnormality in insulin secretion. While the role of glucolipotoxicity in humans is still debated, the inhibitory effects of chronically elevated fatty acid levels has been clearly demonstrated in several studies, at least in individuals genetically predisposed to developing type 2 diabetes. It is therefore likely that glucolipotoxicity contributes to beta-cell failure in type 2 diabetes as well as to the decline in beta-cell function observed after the onset of the disease.
Copyright (c) 2009 Elsevier B.V. All rights reserved.
Figures
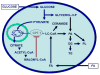
Figure 1. Effects of glucose on lipid partitioning in the beta cell
In the presence of simultaneously elevated levels of glucose and fatty-acid (FA), the increase in cytosolic malonyl-CoA resulting from glucose metabolism inhibits the enzyme carnitine-palmitoyl transferase-1 (CPT-1). Transport of long-chain acyl-CoA (LC-CoA) in the mitochondria is reduced, and the esterification pathway is preferentially activated, leading to cytosolic accumulation of lipid-derived signaling molecules such as ceramide, diglycerides (DG), phosphatidic acid (PA), phospholipids (PL), and triglycerides (TG).
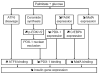
Figure 2. Working model of the mechanisms of fatty-acid inhibition of insulin gene expression
Several signaling pathways are activated in beta cells in the presence of simultaneously elevated levels of palmitate and glucose. First, de novo ceramide synthesis [17] leads to sustained activation of ERK ½ [82] and exclusion of PDX-1 from the nuclear compartment [18]. Second, palmitate blocks glucose-induction of PASK expression, which results in decreased PDX-1 expression and increased C/EBPβ expression [82]. Third, palmitate decreases MafA expression [18]. These 3 pathways result in decreased binding activities of PDX-1 and MafA on the insulin promoter. In addition, palmitate induces the cleavage of ATF6, which also represses insulin gene transcription (our unpublished data).
Figure 3. Concentrations of unbound fatty acids (FA) in solution as a function of the fatty acid: BSA ratio for a fixed total palmitate concentration of 0.5 mM
Unbound fatty acids were measured using the fluorescent probe ADIFAB [114]. Data are the average of 2 independent experiments. Also represented are the mean ± SD of unbound FA levels measured in human plasma using the same method, from [115].
Figure 4. Hypothetical representation of the progression from beta-cell compensation to failure in the face of obesity-induced insulin resistance, and the role of glucolipotoxicity
According to this hypothesis, the decrease in insulin sensitivity is initially matched by a marked increase in insulin secretion, insulin gene expression, and beta-cell mass. At this stage the beta-cell adapts to nutrient oversupply by switching to preferential utilization of fatty acids, as part of the compensatory response (glucolipoadaptation [2]). In genetically predisposed individuals, the beta cell eventually becomes unable to further compensate and glucolipoadaptation evolves towards glucolipotoxicity, in which excursions of blood glucose levels outside of the normal range become permissive for the detrimental effects of elevated fatty acids. This phase is characterized by an early loss of insulin gene expression, decreased insulin secretion (relative to the degree of insulin resistance), and reduced beta-cell mass. Finally, beta-cell failure occurs when glucose levels are permanently in the hyperglycemic range. At that stage both glucotoxicity and glucolipotoxicity contribute to the continued deterioration of beta-cell function.
Similar articles
- Glucolipotoxicity of the pancreatic beta-cell: myth or reality?
Poitout V. Poitout V. Biochem Soc Trans. 2008 Oct;36(Pt 5):901-4. doi: 10.1042/BST0360901. Biochem Soc Trans. 2008. PMID: 18793158 Free PMC article. Review. - Involvement of Per-Arnt-Sim Kinase and extracellular-regulated kinases-1/2 in palmitate inhibition of insulin gene expression in pancreatic beta-cells.
Fontés G, Semache M, Hagman DK, Tremblay C, Shah R, Rhodes CJ, Rutter J, Poitout V. Fontés G, et al. Diabetes. 2009 Sep;58(9):2048-58. doi: 10.2337/db08-0579. Epub 2009 Jun 5. Diabetes. 2009. PMID: 19502418 Free PMC article. - Role of fatty acids in the pathogenesis of ß-cell failure and Type-2 diabetes.
Jiménez-Sánchez C, Oberhauser L, Maechler P. Jiménez-Sánchez C, et al. Atherosclerosis. 2024 Nov;398:118623. doi: 10.1016/j.atherosclerosis.2024.118623. Epub 2024 Oct 4. Atherosclerosis. 2024. PMID: 39389828 Review. - Recent Insights Into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes.
Lytrivi M, Castell AL, Poitout V, Cnop M. Lytrivi M, et al. J Mol Biol. 2020 Mar 6;432(5):1514-1534. doi: 10.1016/j.jmb.2019.09.016. Epub 2019 Oct 16. J Mol Biol. 2020. PMID: 31628942 Free PMC article. Review. - Glucolipotoxicity: fuel excess and beta-cell dysfunction.
Poitout V, Robertson RP. Poitout V, et al. Endocr Rev. 2008 May;29(3):351-66. doi: 10.1210/er.2007-0023. Epub 2007 Nov 29. Endocr Rev. 2008. PMID: 18048763 Free PMC article. Review.
Cited by
- Prediction of antidiabetic effect after gastrectomy with Roux-en-Y reconstruction in patients with gastric cancer and type 2 diabetes.
Seo SH, Cho Y, Heo YS, Seo DH, Ahn SH, Hong SB, Suh YJ, Kim SH. Seo SH, et al. Medicine (Baltimore). 2022 Sep 9;101(36):e30309. doi: 10.1097/MD.0000000000030309. Medicine (Baltimore). 2022. PMID: 36086777 Free PMC article. - Chronic Exposure to Excess Nutrients Left-shifts the Concentration Dependence of Glucose-stimulated Insulin Secretion in Pancreatic β-Cells.
Erion KA, Berdan CA, Burritt NE, Corkey BE, Deeney JT. Erion KA, et al. J Biol Chem. 2015 Jun 26;290(26):16191-201. doi: 10.1074/jbc.M114.620351. Epub 2015 May 1. J Biol Chem. 2015. PMID: 25934392 Free PMC article. - A role for aberrant protein palmitoylation in FFA-induced ER stress and β-cell death.
Baldwin AC, Green CD, Olson LK, Moxley MA, Corbett JA. Baldwin AC, et al. Am J Physiol Endocrinol Metab. 2012 Jun 1;302(11):E1390-8. doi: 10.1152/ajpendo.00519.2011. Epub 2012 Mar 20. Am J Physiol Endocrinol Metab. 2012. PMID: 22436701 Free PMC article. - The Expression of Aldolase B in Islets Is Negatively Associated With Insulin Secretion in Humans.
Gerst F, Jaghutriz BA, Staiger H, Schulte AM, Lorza-Gil E, Kaiser G, Panse M, Haug S, Heni M, Schütz M, Stadion M, Schürmann A, Marzetta F, Ibberson M, Sipos B, Fend F, Fleming T, Nawroth PP, Königsrainer A, Nadalin S, Wagner S, Peter A, Fritsche A, Richter D, Solimena M, Häring HU, Ullrich S, Wagner R. Gerst F, et al. J Clin Endocrinol Metab. 2018 Dec 1;103(12):4373-4383. doi: 10.1210/jc.2018-00791. J Clin Endocrinol Metab. 2018. PMID: 30202879 Free PMC article. - miR-375 enhances palmitate-induced lipoapoptosis in insulin-secreting NIT-1 cells by repressing myotrophin (V1) protein expression.
Li Y, Xu X, Liang Y, Liu S, Xiao H, Li F, Cheng H, Fu Z. Li Y, et al. Int J Clin Exp Pathol. 2010 Jan 25;3(3):254-64. Int J Clin Exp Pathol. 2010. PMID: 20224724 Free PMC article.
References
- U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249–1258. - PubMed
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G. the ADOPT Study Group, Glycemic Durability of Rosiglitazone, Metformin, or Glyburide Monotherapy. N Engl J Med. 2006;355:2427–2443. - PubMed
- Robertson RP, Harmon JS, Tanaka Y, Sacchi G, Tran POT, Gleason CE, Poitout V. Glucose toxicity of the beta-cell: cellular and molecular mechanisms Diabetes Mellitus. In: Le Roith D, Taylor SI, Olefsky JM, editors. A fundamental and clinical text. 2. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 125–132.
Publication types
MeSH terms
Substances
Grants and funding
- MOP 77686/CAPMC/ CIHR/Canada
- 77686/CAPMC/ CIHR/Canada
- R01 DK058096-06/DK/NIDDK NIH HHS/United States
- R01 DK058096-05A2/DK/NIDDK NIH HHS/United States
- R01 DK058096-08/DK/NIDDK NIH HHS/United States
- R01 DK058096-07/DK/NIDDK NIH HHS/United States
- R01-DK58096/DK/NIDDK NIH HHS/United States
- R01 DK058096/DK/NIDDK NIH HHS/United States
- R56 DK058096-05/DK/NIDDK NIH HHS/United States
- R56 DK058096/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous