Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2 - PubMed (original) (raw)
Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2
Ha Won Kim et al. J Biol Chem. 2009.
Abstract
MicroRNAs (miRs) participate in most cellular functions by posttranscriptional regulation of gene expression albeit with little information regarding their role in ischemic preconditioning (IP) of stem cells. We report that IP of bone marrow-derived mesenchymal stem cells (MSCs) with two cycles of 30-min ischemia/reoxygenation (I/R) supported their survival under subsequent longer exposure to anoxia and following engraftment in the infarcted heart. IP significantly reduced apoptosis in MSCs through activation of Akt (Ser(473)) and ERK1/2 (Thr(202)/Tyr(204)) and nuclear translocation of hypoxia-inducible factor-1alpha (HIF-1alpha). We observed concomitant induction of miR-210 in the preconditioned MSCs ((PC)MSCs). Inhibition of HIF-1alpha or of miR-210 abrogated the cytoprotective effects of preconditioning. Extrapolation of these data to in vivo studies in a rat model of acute myocardial infarction predominantly improved stem cell survival after engraftment with a role for miR-210. Notably, multiple I/R cycles more effectively regulated the miR-210 and hence promoted MSC survival compared with single-cycle hypoxia of an equal duration. Real time PCR array for rat apoptotic genes, computational target gene analyses, and luciferase reporter assay identified FLICE-associated huge protein (FLASH)/caspase-8-associated protein-2 (Casp8ap2) in (PC)MSCs as the target gene of miR-210. Induction of FLASH/CASP8AP2 in miR-210 knocked-down (PC)MSCs resulted in increased cell apoptosis. Taken together, these data demonstrated that cytoprotection afforded by IP was regulated by miR-210 induction via FLASH/Casp8ap2 suppression. These results highlighted that IP by multiple short episodes of I/R is a novel strategy to promote stem cell survival.
Figures
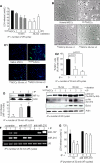
FIGURE 1.
A, IP attenuated cellular injury as examined by LDH release subsequent to 6 h of lethal anoxia. IP with three 30-min cycles was less effective than IP using one 30-min cycle and two 30-min cycles (* and ** versus all other groups of cells, p < 0.05). B, morphological changes of MSCs by 6 h of anoxia included rounding off and shrinkage of cells, which was prevented by IP (magnification, ×200). C1 and C2, representative fluorescence images and quantitative analysis of TUNEL positivity showing decreased number of TUNEL+ cells in PCMSCs (p < 0.01 versus non-PCMSCs) (magnification, ×200; green, TUNEL+ nuclei; blue, DAPI). D, Western blot showing higher HIF-1α in nuclear fraction (two cycles of 30-min I/R) compared with PCMSCs (one cycle of 30-min I/R) and non-PCMSCs. (Ť and T̄ vs. Ψ, p < 0.05). E, Western blot showing higher activation of ERK1/2, Akt, and Bcl.xL in PCMSCs (two cycles of 30-min I/R) compared with PCMSCs (one cycle of 30-min I/R) and non-PCMSCs. F, RT-PCR showing successful and specific knockdown of endogenous miR-210 and miR-107 by transfection with antisense molecules specific for respective miRs. Knock down of miR-107 was used as a control to show the specificity antisense interference. G, abrogation of miR-210 led to loss of cytoprotection by IP in MSCs as determined by LDH release assay. Abrogation of miR-210 completely abolished IP-induced cytoprotection (T̄ and Ť versus all other groups of cells, p < 0.05).
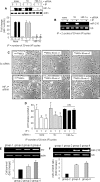
FIGURE 2.
A, Western blot showing successful abrogation of IP-induced HIF-1α in the nuclear fraction of PCMSCs transfected with HIF-1α-specific siRNA (T̄ & Ť versus all other groups, p < 0. 01). B, abrogation of IP-induced miR-210 was observed in PCMSCs transfected with HIF-1α-specific siRNA showing their dependence on HIF-1α. C and D, abrogation of HIF-1α and miR-210 resulted in a loss of morphologic integrity (magnification, ×200) and IP-induced cytoprotection as determined by LDH assay (Ť versus T̄, p < 0.05). NS, not significant. E, PCR for sry gene of male donor cells in the female recipient rat heart on day 4 after cell transplantation. A higher survival of transplanted cells was observed in PCMSC groups 3 and 4 (p < 0.05 versus non-PCMSCs). PCMSC survival was higher in group 4 compared with group 3 (p < 0.05). No sry gene was observed in the Dulbecco's modified Eagle's medium-injected female hearts. F, expression of miR-210 in groups 3 and 4 animal hearts was higher compared with group 2 (p < 0.05).
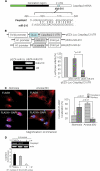
FIGURE 3.
miR-210 targets Casp8ap2, a positive regulator of apoptosis. A, one putative target site of miR-210 highly conserved in the Casp8ap2 mRNA 3′-UTR. B, construction of pEZX-Luc-Casp8ap2 3′-UTR luciferase reporter plasmid and precursor miR-210 expression clone. qRT-PCR showed successful transfection and significantly higher expression of miR-210 in MSCs using pEZX-miR-210 plasmid compared with pEZX-miR-Sc plasmid-transfected MSCs. Cotransfection of MSCs with pEZX-Luc vector containing Casp8ap2 3′-UTR together with a plasmid encoding miR-210 showed decreased luciferase activity (p < 0.01 versus pEZX-miR-Sc-transfected cells). The ratio of luciferase activity was calculated either in the presence or absence of miR-210. C, immunofluorescence staining of MSCs stained for FLASH/Casp8ap2 (red; indicated by white arrows) and its overlay images of nuclei stained with DAPI (blue). Compared with its nuclear localization under normoxia, anoxia translocated FLASH/CASP8AP2 from the nucleus into the cytoplasm as indicated by lack of red fluorescence in the nuclei. The percentage of cells (at ×40) with FLASH/CASP8AP2-positive nuclei was significantly higher in the cells cultured under normoxia (p < 0.01 versus cells treated with anoxia for 6 h). D, RT-PCR showing the effect of preconditioning on Casp8ap2 gene expression in MSCs under 6 h of anoxia. Casp8ap2 gene expression was significantly abolished in MSCs preconditioned by two 30-min cycles of I/R compared with non-PCMSCs and MSCs preconditioned by 1 cycle of I/R (* versus other groups, p < 0.01).
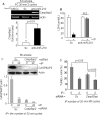
FIGURE 4.
A, qRT-PCR for Casp8ap2 gene expression in PCMSCs exposed to 6 h of anoxia. Casp8ap2 gene expression showed a significant increase in miR-210 knock-down cells compared with native (nontransfected) and Sc siRNA-transfected MSCs. B, LDH release was significantly increased in PCMSCs with miR-210 knock-down cells compared with Sc siRNA-transfected MSCs after 6 h of anoxia (*, p < 0.01 versus non-PCMSCs). C, Western blot showing successful abrogation of FLASH/CASP8AP2 in both non-PCMSCs and PCMSCs transfected with Casp8ap2 siRNA compared with Sc siRNA-transfected non-PCMSCs. PCMSCs transfected with Sc siRNA had very low expression of CASP8AP2, thus implying that preconditioning down-regulated Casp8ap2 expression. D, TUNEL-positive MSCs were significantly reduced in PCMSCs and non-PCMSCs transfected with Casp8ap2 siRNA compared with Sc siRNA-transfected cells after 6 h of anoxia.
Similar articles
- Concomitant activation of miR-107/PDCD10 and hypoxamir-210/Casp8ap2 and their role in cytoprotection during ischemic preconditioning of stem cells.
Kim HW, Mallick F, Durrani S, Ashraf M, Jiang S, Haider KH. Kim HW, et al. Antioxid Redox Signal. 2012 Oct 15;17(8):1053-65. doi: 10.1089/ars.2012.4518. Epub 2012 May 23. Antioxid Redox Signal. 2012. PMID: 22482882 Free PMC article. - Stem cell-based delivery of Hypoxamir-210 to the infarcted heart: implications on stem cell survival and preservation of infarcted heart function.
Kim HW, Jiang S, Ashraf M, Haider KH. Kim HW, et al. J Mol Med (Berl). 2012 Sep;90(9):997-1010. doi: 10.1007/s00109-012-0920-1. Epub 2012 May 31. J Mol Med (Berl). 2012. PMID: 22648522 Free PMC article. - LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning.
Meng SS, Xu XP, Chang W, Lu ZH, Huang LL, Xu JY, Liu L, Qiu HB, Yang Y, Guo FM. Meng SS, et al. Stem Cell Res Ther. 2018 Oct 25;9(1):280. doi: 10.1186/s13287-018-1031-x. Stem Cell Res Ther. 2018. PMID: 30359325 Free PMC article. - Preconditioning and stem cell survival.
Haider HKh, Ashraf M. Haider HKh, et al. J Cardiovasc Transl Res. 2010 Apr;3(2):89-102. doi: 10.1007/s12265-009-9161-2. Epub 2009 Dec 22. J Cardiovasc Transl Res. 2010. PMID: 20560023 Review. - Preconditioning of MSCs for Acute Neurological Conditions: From Cellular to Functional Impact-A Systematic Review.
Serrenho I, Ferreira SA, Baltazar G. Serrenho I, et al. Cells. 2024 May 16;13(10):845. doi: 10.3390/cells13100845. Cells. 2024. PMID: 38786067 Free PMC article. Review.
Cited by
- HIF-1α Overexpression Induces Angiogenesis in Mesenchymal Stem Cells.
Razban V, Lotfi AS, Soleimani M, Ahmadi H, Massumi M, Khajeh S, Ghaedi M, Arjmand S, Najavand S, Khoshdel A. Razban V, et al. Biores Open Access. 2012 Aug;1(4):174-83. doi: 10.1089/biores.2012.9905. Biores Open Access. 2012. PMID: 23514846 Free PMC article. - miR-210: the master hypoxamir.
Chan YC, Banerjee J, Choi SY, Sen CK. Chan YC, et al. Microcirculation. 2012 Apr;19(3):215-23. doi: 10.1111/j.1549-8719.2011.00154.x. Microcirculation. 2012. PMID: 22171547 Free PMC article. Review. - Preconditioning strategy in stem cell transplantation therapy.
Yu SP, Wei Z, Wei L. Yu SP, et al. Transl Stroke Res. 2013 Feb;4(1):76-88. doi: 10.1007/s12975-012-0251-0. Transl Stroke Res. 2013. PMID: 23914259 Free PMC article. Review. - Survival of hypoxic human mesenchymal stem cells is enhanced by a positive feedback loop involving miR-210 and hypoxia-inducible factor 1.
Chang W, Lee CY, Park JH, Park MS, Maeng LS, Yoon CS, Lee MY, Hwang KC, Chung YA. Chang W, et al. J Vet Sci. 2013;14(1):69-76. doi: 10.4142/jvs.2013.14.1.69. Epub 2013 Feb 5. J Vet Sci. 2013. PMID: 23388440 Free PMC article.
References
- Kloosterman W. P., Plasterk R. H. (2006) Dev. Cell 11, 441–450 - PubMed
- Ambros V. (2004) Nature 431, 350–355 - PubMed
- Foshay K. M., Gallicano G. I. (2007) Curr. Stem Cell Res. Ther. 2, 264–271 - PubMed
- Kulshreshtha R., Davuluri R. V., Calin G. A., Ivan M. (2008) Cell Death Differ. 15, 667–671 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL089535/HL/NHLBI NIH HHS/United States
- HL087246/HL/NHLBI NIH HHS/United States
- R01 HL087288/HL/NHLBI NIH HHS/United States
- R01 HL087246/HL/NHLBI NIH HHS/United States
- R37-HL074272/HL/NHLBI NIH HHS/United States
- HL-080686/HL/NHLBI NIH HHS/United States
- R01 HL080686/HL/NHLBI NIH HHS/United States
- R37 HL074272/HL/NHLBI NIH HHS/United States
- HL089535/HL/NHLBI NIH HHS/United States
- HL087288/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous