The p53 tumor suppressor protein is a critical regulator of hematopoietic stem cell behavior - PubMed (original) (raw)
Review
The p53 tumor suppressor protein is a critical regulator of hematopoietic stem cell behavior
Yan Liu et al. Cell Cycle. 2009.
Abstract
In response to diverse stresses, the tumor suppressor p53 differentially regulates its target genes, variably inducing cell-cycle arrest, apoptosis or senescence. Emerging evidence indicates that p53 plays an important role in regulating hematopoietic stem cell (HSC) quiescence, self-renewal, apoptosis and aging. The p53 pathway is activated by DNA damage, defects in ribosome biogenesis, oxidative stress and oncogene induced p19 ARF upregulation. We present an overview of the current state of knowledge about p53 (and its target genes) in regulating HSC behavior, with the hope that understanding the molecular mechanisms that control p53 activity in HSCs and how p53 mutations affect its role in these events may facilitate the development of therapeutic strategies for eliminating leukemia (and cancer) propagating cells.
Figures
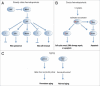
Figure 1
p53 regulates hematopoietic stem cell behavior. (A) The role of p53 in steady state hematopoiesis. During steady state, p53 plays an important role in regulating both hematopoietic stem cell (HSC) quiescence and self-renewal. p53 normally maintains HSCs in a quiescent state, and in its absence, HSCs are driven into the cell cycle. The transcription factor MEF/ELF4 can directly regulate Mdm2 expression, thus MEF null mice have enhanced p53 activity. Loss of p53 in MEF/ELF4 null HSCs leads to normal HSC quiescence demonstrating that p53 is essential for the enhanced HSC quiescence seen in the absence of MEF. Both Gfi-1 and Necdin are p53 target genes and both function to restrict the proliferation of HSC. p53 has been shown to limit HSC self-renewal, yet how p53 regulates this process is unknown. (B) The role of p53 in stress hematopoiesis. In response to diverse stresses, p53 differentially regulates its target genes, variably inducing cell cycle arrest, apoptosis or senescence. p21 is a major target of p53 under stress conditions, and p21 has been implicated in cell cycle arrest, DNA damage repair and apoptosis. Slug is transcriptionally induced by p53 following irradiation and Slug protects the damaged cells from apoptosis by directly repressing p53-mediated activation of Puma gene expression. (C) The role of p53 in hematopoietic stem cell aging. p53 has been implicated in the aging process, as p53 hypermorphic (p53+/m) mice have higher p53 activity than wild-type mice, and display premature aging. However, “super-p53” mice, which have three copies of the wild type p53 gene, have a normal lifespan and show no evidence of early aging. It seems that the constitutively high p53 activity in the p53+/m mice accelerates aging, while the extra copy of p53 in the “super-p53” mice is subject to normal regulation, and may not lead to a change in p53 functional activity.
Similar articles
- The p53 tumor suppressor protein regulates hematopoietic stem cell fate.
Asai T, Liu Y, Bae N, Nimer SD. Asai T, et al. J Cell Physiol. 2011 Sep;226(9):2215-21. doi: 10.1002/jcp.22561. J Cell Physiol. 2011. PMID: 21660944 Free PMC article. Review. - The ups and downs of p53 regulation in hematopoietic stem cells.
Abbas HA, Pant V, Lozano G. Abbas HA, et al. Cell Cycle. 2011 Oct 1;10(19):3257-62. doi: 10.4161/cc.10.19.17721. Epub 2011 Oct 1. Cell Cycle. 2011. PMID: 21957490 Free PMC article. - p53 regulates hematopoietic stem cell quiescence.
Liu Y, Elf SE, Miyata Y, Sashida G, Liu Y, Huang G, Di Giandomenico S, Lee JM, Deblasio A, Menendez S, Antipin J, Reva B, Koff A, Nimer SD. Liu Y, et al. Cell Stem Cell. 2009 Jan 9;4(1):37-48. doi: 10.1016/j.stem.2008.11.006. Cell Stem Cell. 2009. PMID: 19128791 Free PMC article. - Fine-tuning p53 activity through C-terminal modification significantly contributes to HSC homeostasis and mouse radiosensitivity.
Wang YV, Leblanc M, Fox N, Mao JH, Tinkum KL, Krummel K, Engle D, Piwnica-Worms D, Piwnica-Worms H, Balmain A, Kaushansky K, Wahl GM. Wang YV, et al. Genes Dev. 2011 Jul 1;25(13):1426-38. doi: 10.1101/gad.2024411. Genes Dev. 2011. PMID: 21724834 Free PMC article. - A new twist in the feedback loop: stress-activated MDM2 destabilization is required for p53 activation.
Stommel JM, Wahl GM. Stommel JM, et al. Cell Cycle. 2005 Mar;4(3):411-7. doi: 10.4161/cc.4.3.1522. Epub 2005 Mar 2. Cell Cycle. 2005. PMID: 15684615 Review.
Cited by
- Loss of p53 in mesenchymal stem cells promotes alteration of bone remodeling through negative regulation of osteoprotegerin.
Velletri T, Huang Y, Wang Y, Li Q, Hu M, Xie N, Yang Q, Chen X, Chen Q, Shou P, Gan Y, Candi E, Annicchiarico-Petruzzelli M, Agostini M, Yang H, Melino G, Shi Y, Wang Y. Velletri T, et al. Cell Death Differ. 2021 Jan;28(1):156-169. doi: 10.1038/s41418-020-0590-4. Epub 2020 Jul 21. Cell Death Differ. 2021. PMID: 32694652 Free PMC article. - Targeting p53-deficient chronic lymphocytic leukemia cells in vitro and in vivo by ROS-mediated mechanism.
Liu J, Chen G, Pelicano H, Liao J, Huang J, Feng L, Keating MJ, Huang P. Liu J, et al. Oncotarget. 2016 Nov 1;7(44):71378-71389. doi: 10.18632/oncotarget.12110. Oncotarget. 2016. PMID: 27655686 Free PMC article. - Schlafen2 is a regulator of quiescence in adult murine hematopoietic stem cells.
Warsi S, Dahl M, Smith EMK, Rydstrom A, Mansell E, Sigurdsson V, Sjoberg J, Soneji S, Rorby E, Siva K, Grahn THM, Liu Y, Blank U, Karlsson G, Karlsson S. Warsi S, et al. Haematologica. 2022 Dec 1;107(12):2884-2896. doi: 10.3324/haematol.2021.279799. Haematologica. 2022. PMID: 35615926 Free PMC article. - Dual inhibition of MDMX and MDM2 as a therapeutic strategy in leukemia.
Carvajal LA, Neriah DB, Senecal A, Benard L, Thiruthuvanathan V, Yatsenko T, Narayanagari SR, Wheat JC, Todorova TI, Mitchell K, Kenworthy C, Guerlavais V, Annis DA, Bartholdy B, Will B, Anampa JD, Mantzaris I, Aivado M, Singer RH, Coleman RA, Verma A, Steidl U. Carvajal LA, et al. Sci Transl Med. 2018 Apr 11;10(436):eaao3003. doi: 10.1126/scitranslmed.aao3003. Sci Transl Med. 2018. PMID: 29643228 Free PMC article. - Network Pharmacology-Based Investigation of the System-Level Molecular Mechanisms of the Hematopoietic Activity of Samul-Tang, a Traditional Korean Herbal Formula.
Lee HS, Lee IH, Park SI, Lee DY. Lee HS, et al. Evid Based Complement Alternat Med. 2020 Feb 13;2020:9048089. doi: 10.1155/2020/9048089. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32104198 Free PMC article.
References
- Attar EC, Scadden DT. Regulation of hematopoietic stem cell growth. Leukemia. 2004;18:1760–8. - PubMed
- Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–86. - PubMed
- Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 DK052208/DK/NIDDK NIH HHS/United States
- R56 DK052208/DK/NIDDK NIH HHS/United States
- R01 DK52208/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous