Gasp, a Grb2-associating protein, is critical for positive selection of thymocytes - PubMed (original) (raw)
. 2009 Sep 22;106(38):16345-50.
doi: 10.1073/pnas.0908593106. Epub 2009 Sep 14.
Hiroyo Oda, Kunihiro Hayakawa, Yoshinori Sato, Koji Eshima, Teruo Kirikae, Shun-Ichiro Iemura, Mutsunori Shirai, Takaya Abe, Tohru Natsume, Takehiko Sasazuki, Harumi Suzuki
Affiliations
- PMID: 19805304
- PMCID: PMC2752560
- DOI: 10.1073/pnas.0908593106
Gasp, a Grb2-associating protein, is critical for positive selection of thymocytes
Michael S Patrick et al. Proc Natl Acad Sci U S A. 2009.
Abstract
T cells develop in the thymus through positive and negative selection, which are responsible for shaping the T cell receptor (TCR) repertoire. To elucidate the molecular mechanisms involved in selection remains an area of intense interest. Here, we identified and characterized a gene product Gasp (Grb2-associating protein, also called Themis) that is critically required for positive selection. Gasp is a cytosolic protein with no known functional motifs that is expressed only in T cells, especially immature CD4/CD8 double positive (DP) thymocytes. In the absence of Gasp, differentiation of both CD4 and CD8 single positive cells in the thymus was severely inhibited, whereas all other TCR-induced events such as beta-selection, negative selection, peripheral activation, and homeostatic proliferation were unaffected. We found that Gasp constitutively associates with Grb2 via its N-terminal Src homology 3 domain, suggesting that Gasp acts as a thymocyte-specific adaptor for Grb2 or regulates Ras signaling in DP thymocytes. Collectively, we have described a gene called Gasp that is critical for positive selection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
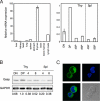
Thymus-specific expression of Gasp. (A) Expression of Gasp mRNA was analyzed by real-time PCR from C57BL/6 mouse tissues and cells. RNA expression was normalized relative to β-actin. Error bars are the SD (n = 2 and 3). (B) Expression of Gasp protein in sorted thymic or splenic T cell subpopulations. Results are representative of two independent experiments. (C) Representative micrograph showing subcellular localization of Gasp. GFP-fused Gasp was exogenously introduced in DPK cell line (green) and costained with DAPI (blue). (Magnification: ×1,000.)
Fig. 2.
Generation and analysis of _Gasp_−/−mice. (A) A gene-targeting strategy was used to insert a lacZ/neo cassette into exon 1 of the Gasp gene. (B) Western blot analysis of DP thymocytes with anti-Gasp-specific antisera. Results are representative of two independent experiments. (C) CD4 and CD8 profile of Gasp+/+, Gasp+/−, and _Gasp_−/− thymocytes. (D) CD4 and CD8 expression of neonatal thymocytes. (E) CD4 and CD8 profile of thymocytes from OT-I, OT-II Tg _RAG_−/−, and female HY Tg _RAG_−/−mice. (F) CD4 and CD8 profile and number of thymocytes from male HY Tg _RAG_−/−mice. In C–F, data are representative of more than three independent experiments.
Fig. 3.
Phenotype of peripheral T cells in _Gasp_−/−mice. (A) CD4 and CD8 profile of the cells from spleen, peripheral blood (PBL), mLN, and iLN of Gasp+/+ and _Gasp_−/− mice. Results are representative of more than five independent experiments. (B) Absolute number of CD4-SP and CD8-SP cells of Spl and mLN of Gasp+/+ and _Gasp_−/− mice. Each dot represents individual mice at day of age. Solid (−/−) and dashed (+/+) lines show the linear regression correlation between age and absolute number of cells. (C) CD44 and CD62L profile of splenic CD4-SP and CD8-SP cells from Gasp+/+ and _Gasp_−/− mice. (D) BrdU uptake of CD4-SP and CD8-SP cells from spleen and mLN of Gasp+/+ and _Gasp_−/− mice. After mice were fed with BrdU for 5 days with drinking water, the cells were stained with anti-BrdU antibody. In C and D, results are representative of more than three independent experiments.
Fig. 4.
Activation of peripheral CD4 and CD8 mature T cells. (A) MTT assay was applied for TCR-stimulated sorted splenic CD4-SP cells of Gasp+/+ and _Gasp_−/− mice after 3 days of culture. (B) Primary CD4-SP T cells from Gasp+/+ and _Gasp_−/− mice were stimulated with plate-bound anti-CD3 or CD3+ 28 mAb for 24 h, and IL-2 concentration in supernatants was measured by ELISA. (C) TCR-dependent production of IL4 and IFN-γ from cell line established from Gasp+/+ and _Gasp_−/− splenocyte. (D) H-2d-specific allo-CTL lines were established from splenic CD8-SP cells, and CTL assay was analyzed for CTL function by using specific (P815) and nonspecific (EL4). Results are representative of more than two independent experiments.
Fig. 5.
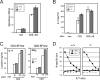
Characteristics of Gasp deficiency. (A) Defects in _Gasp_−/− mice are independent of PTPRK. Expression of PTPRK mRNA in _Gasp_−/− thymocytes was determined by real-time RT-PCR. Error bars are the SD (n = 2 and 3). (B) Developmental defects in _Gasp_−/− mice are thymocyte intrinsic. Bone marrow cells from CD45.1 Gasp+/+ mice were injected into lethally irradiated CD45.2 _Gasp_−/− mice or vice versa. After 2 months, cells from indicated organs were stained with CD4, 8, 45.1, and 45.2. Results are representative of more than two independent mice. (C) Proportion of post-selected CD69+ TCRhi DP cells in Gasp+/+ and _Gasp_−/− mice. Results are representative of four independent experiments. (D) Sorted DP thymocytes from Gasp+/+ and _Gasp_−/−mice were activated with plate-bound CD3 + 28 Ab overnight, then stained with CD69. Results are representative of more than three independent experiments. (E) DP cells from _Gasp_−/− mice were stimulated with anti-CD3 mAb followed by anti-hamster IgG, then Ca2+ concentration was measured by using Fura2-AM. Results are representative of three independent experiments. (F) DP thymocytes were activated by the indicated stimuli (2 min for anti-CD3 and 3 + 28, 5 min for PMA+Iono), then Western blotted with phosphorylated-ERK-, SLP76-, and PLCγ-specific antibody. Results are representative of more than three independent experiments.
Fig. 6.
Gasp associates with Grb2. (A) HEK293T cells were transfected with myc-tagged Grb2, SH2-deleted Grb2 (Grb2-SH2-myc), N-/C-terminal SH3 mutant (Grb2–49L/203R-myc), and Flag-tagged Gasp. Lysates were immunoprecipitated with anti-Flag mAb then blotted with the indicated Abs. (B) myc-Grb2, Grb2–49L/203R, C-terminal SH3 mutant (Grb2–203R-myc), and Flag-Gasp were transfected and immunoprecipitated with anti-myc mAb then blotted with the indicated Abs. Results are representative of more than seven independent experiments.
Similar articles
- Transgenic expression of RasGRP1 induces the maturation of double-negative thymocytes and enhances the production of CD8 single-positive thymocytes.
Norment AM, Bogatzki LY, Klinger M, Ojala EW, Bevan MJ, Kay RJ. Norment AM, et al. J Immunol. 2003 Feb 1;170(3):1141-9. doi: 10.4049/jimmunol.170.3.1141. J Immunol. 2003. PMID: 12538669 - Constitutive Notch signalling promotes CD4 CD8 thymocyte differentiation in the absence of the pre-TCR complex, by mimicking pre-TCR signals.
Michie AM, Chan AC, Ciofani M, Carleton M, Lefebvre JM, He Y, Allman DM, Wiest DL, Zúñiga-Pflücker JC, Izon DJ. Michie AM, et al. Int Immunol. 2007 Dec;19(12):1421-30. doi: 10.1093/intimm/dxm113. Epub 2007 Nov 1. Int Immunol. 2007. PMID: 17981791 - Grb2-Mediated Recruitment of USP9X to LAT Enhances Themis Stability following Thymic Selection.
Garreau A, Blaize G, Argenty J, Rouquié N, Tourdès A, Wood SA, Saoudi A, Lesourne R. Garreau A, et al. J Immunol. 2017 Oct 15;199(8):2758-2766. doi: 10.4049/jimmunol.1700566. Epub 2017 Sep 6. J Immunol. 2017. PMID: 28877990 - Positive and negative thymocyte selection.
Saito T, Watanabe N. Saito T, et al. Crit Rev Immunol. 1998;18(4):359-70. doi: 10.1615/critrevimmunol.v18.i4.40. Crit Rev Immunol. 1998. PMID: 9704194 Review. - Tenuous paths in unexplored territory: From T cell receptor signaling to effector gene expression during thymocyte selection.
Wang L, Xiong Y, Bosselut R. Wang L, et al. Semin Immunol. 2010 Oct;22(5):294-302. doi: 10.1016/j.smim.2010.04.013. Semin Immunol. 2010. PMID: 20537906 Free PMC article. Review.
Cited by
- T-cell tolerance: central and peripheral.
Xing Y, Hogquist KA. Xing Y, et al. Cold Spring Harb Perspect Biol. 2012 Jun 1;4(6):a006957. doi: 10.1101/cshperspect.a006957. Cold Spring Harb Perspect Biol. 2012. PMID: 22661634 Free PMC article. - THEMIS-tery is solved.
Wiest DL. Wiest DL. Nat Immunol. 2017 Mar 22;18(4):368-370. doi: 10.1038/ni.3708. Nat Immunol. 2017. PMID: 28323266 No abstract available. - THEMIS promotes T cell development and maintenance by rising the signaling threshold of the inhibitory receptor BTLA.
Mélique S, Vadel A, Rouquié N, Yang C, Bories C, Cotineau C, Saoudi A, Fazilleau N, Lesourne R. Mélique S, et al. Proc Natl Acad Sci U S A. 2024 May 14;121(20):e2318773121. doi: 10.1073/pnas.2318773121. Epub 2024 May 7. Proc Natl Acad Sci U S A. 2024. PMID: 38713628 Free PMC article. - Tespa1 is a novel inositol 1,4,5-trisphosphate receptor binding protein in T and B lymphocytes.
Matsuzaki H, Fujimoto T, Ota T, Ogawa M, Tsunoda T, Doi K, Hamabashiri M, Tanaka M, Shirasawa S. Matsuzaki H, et al. FEBS Open Bio. 2012 Sep 4;2:255-9. doi: 10.1016/j.fob.2012.08.005. Print 2012. FEBS Open Bio. 2012. PMID: 23650607 Free PMC article. - The Ras GTPase-activating protein Rasal3 supports survival of naive T cells.
Muro R, Nitta T, Okada T, Ideta H, Tsubata T, Suzuki H. Muro R, et al. PLoS One. 2015 Mar 20;10(3):e0119898. doi: 10.1371/journal.pone.0119898. eCollection 2015. PLoS One. 2015. PMID: 25793935 Free PMC article.
References
- Rothenberg EV, Taghon T. Molecular genetics of T cell development. Annu Rev Immunol. 2005;23:601–649. - PubMed
- Suzuki H, Punt JA, Granger LG, Singer A. Asymmetric signaling requirements for thymocyte commitment to the CD4+ versus CD8+ T cell lineages: A new perspective on thymic commitment and selection. Immunity. 1995;2:413–425. - PubMed
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. - PubMed
- Germain RN. Ligand-dependent regulation of T cell development and activation. Immuol Res. 2003;27:277–286. - PubMed
- Alberola-Ila J, Hernandez-Hoyos G. The Ras/MAPK cascade and the control of positive selection. Immunol Rev. 2003;191:79–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous