Chemical library screens targeting an HIV-1 accessory factor/host cell kinase complex identify novel antiretroviral compounds - PubMed (original) (raw)
Chemical library screens targeting an HIV-1 accessory factor/host cell kinase complex identify novel antiretroviral compounds
Lori Emert-Sedlak et al. ACS Chem Biol. 2009.
Abstract
Nef is an HIV-1 accessory protein essential for AIDS progression and an attractive target for drug discovery. Lack of a catalytic function makes Nef difficult to assay in chemical library screens. We developed a high-throughput screening assay for inhibitors of Nef function by coupling it to one of its host cell binding partners, the Src-family kinase Hck. Hck activation is dependent upon Nef in this assay, providing a direct readout of Nef activity in vitro. Using this screen, a unique diphenylfuropyrimidine was identified as a strong inhibitor of Nef-dependent Hck activation. This compound also exhibited remarkable antiretroviral effects, blocking Nef-dependent HIV replication in cell culture. Structurally related analogs were synthesized and shown to exhibit similar Nef-dependent antiviral activity, identifying the diphenylfuropyrimidine substructure as a new lead for antiretroviral drug development. This study demonstrates that coupling noncatalytic HIV accessory factors with host cell target proteins addressable by high-throughput assays may afford new avenues for the discovery of anti-HIV agents.
Figures
Figure 1
Screening for Nef:Hck inhibitors using the Z’-Lyte assay. (a) Nef stimulates Hck protein-tyrosine kinase activity in vitro. Recombinant Hck was purified from Sf9 insect cells in its downregulated form (Hck-YEEI; see text) and assayed with a FRET-peptide substrate and ATP as described under Materials and Methods. Reactions were run in the presence of increasing amounts of Hck-YEEI either alone (open circles) or in the presence of a 10-fold molar excess of purified recombinant HIV-1 Nef (closed circles). Each condition was repeated in quadruplicate, and the extent of phosphorylation is expressed as mean percent of phosphorylation relative to a control phosphopeptide ± S.D. Chemical library screens were performed under conditions where Hck-YEEI activation is dependent upon Nef (arrow). (b) Chemical libraries (10,000 compounds total) were screened for inhibitors of Nef-induced Hck kinase activity. Shown are representative emission ratios for 350 compounds from one plate which includes a hit (DFP-4AP; see text for details). The average emission ratio and 50% inhibition cutoff are indicated by the horizontal lines. Clusters of control points include wells with no ATP (open circles) and DMSO vehicle control (open triangles).
Figure 2
Inhibition of Nef-induced Hck activation and HIV-1 replication by DFP-4-aminopropanol (DFP-4AP). (a) Nef-dependence of HIV-1 replication in U87MG cells. Cells were infected with wild-type HIV strain NL4-3 (WT) or a mutant that fails to express Nef (ΔNef) over the range of viral titers shown. Relative HIV p24 levels were determined by ELISA 5 days later. (b) Inhibition of Nef-induced Hck activation by DFP-4AP. The Nef:Hck complex was assayed in vitro with a peptide substrate in the presence of DFP-4AP over the range of concentrations shown. Each concentration was assayed in triplicate and data are expressed as percent inhibition relative to control reactions run in the presence of solvent only. The data were best-fit by non-linear regression analysis (GraphPad Prism Software), yielding an IC50 value of 4 µM. (c) Inhibition of HIV-1 replication by DFP. U87MG cells were infected with HIV strain NL4-3 in the presence of DFP-4AP over the range of concentrations shown. Release of viral p24 was determined by ELISA 5 days later. The data were best-fit by non-linear regression analysis, yielding an IC50 value of 6 µM.
Figure 3
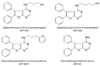
Structures of DFP-4-aminopropanol and related 4-amino derivatives.
Figure 4
Selective inhibition of Nef-induced Hck activation by diphenylfuropyrimidines. The kinase activities of the Nef:Hck complex and Hck alone were assayed in vitro with a peptide substrate in the presence of DFP-4AP and the three analogs shown in Figure 3 over the range of concentrations shown. Each concentration was assayed in triplicate and data are expressed as percent inhibition relative to control reactions run in the absence of compound. Where possible, non-linear regression analysis was used to estimate IC50 values shown (GraphPad Prism).
Figure 5
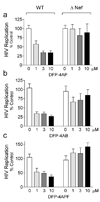
Inhibition of HIV-1 replication by diphenylfuropyrimidines requires Nef. U87MG cells were infected in 96-well plates (200 µl final volume) with equal titers (100 pg p24) of wild-type HIV strain NL4-3 (WT) or a mutant that fails to express Nef (ΔNef) in the presence of the DFP analogs (Figure 3) over the range of concentrations shown. HIV p24 levels were determined by ELISA 5 days later. Data are presented as percent of p24 release observed in the absence of compound ± S.D.
Figure 6
DFP analogs fail to inhibit HIV-1 replication in Jurkat cells. (a) HIV-1 replication is Nef-independent in Jurkat T-cells. Cells were infected in 96-well plates (200 µl final volume) with wild-type HIV strain NL4-3 (WT) or a mutant that fails to express Nef (ΔNef) over the range of viral titers shown. Relative HIV p24 levels were determined by ELISA 5 days later. (b) Jurkat cells were infected with equal titers of wild-type HIV (WT) or the Nef-defective mutant (ΔNef) in the presence of 5 µM of the DFP analogs (Figure 3). HIV p24 release was determined by ELISA 5 days later. Data are presented as percent of p24 release observed in the absence of compound ± S.D.
Similar articles
- Effector kinase coupling enables high-throughput screens for direct HIV-1 Nef antagonists with antiretroviral activity.
Emert-Sedlak LA, Narute P, Shu ST, Poe JA, Shi H, Yanamala N, Alvarado JJ, Lazo JS, Yeh JI, Johnston PA, Smithgall TE. Emert-Sedlak LA, et al. Chem Biol. 2013 Jan 24;20(1):82-91. doi: 10.1016/j.chembiol.2012.11.005. Chem Biol. 2013. PMID: 23352142 Free PMC article. - Nef alleles from all major HIV-1 clades activate Src-family kinases and enhance HIV-1 replication in an inhibitor-sensitive manner.
Narute PS, Smithgall TE. Narute PS, et al. PLoS One. 2012;7(2):e32561. doi: 10.1371/journal.pone.0032561. Epub 2012 Feb 29. PLoS One. 2012. PMID: 22393415 Free PMC article. - Subtle Dynamic Changes Accompany Hck Activation by HIV-1 Nef and are Reversed by an Antiretroviral Kinase Inhibitor.
Wales TE, Hochrein JM, Morgan CR, Emert-Sedlak LA, Smithgall TE, Engen JR. Wales TE, et al. Biochemistry. 2015 Oct 20;54(41):6382-91. doi: 10.1021/acs.biochem.5b00875. Epub 2015 Oct 6. Biochemistry. 2015. PMID: 26440750 Free PMC article. - Structure, function, and inhibitor targeting of HIV-1 Nef-effector kinase complexes.
Staudt RP, Alvarado JJ, Emert-Sedlak LA, Shi H, Shu ST, Wales TE, Engen JR, Smithgall TE. Staudt RP, et al. J Biol Chem. 2020 Oct 30;295(44):15158-15171. doi: 10.1074/jbc.REV120.012317. Epub 2020 Aug 29. J Biol Chem. 2020. PMID: 32862141 Free PMC article. Review. - Strategies for inhibiting function of HIV-1 accessory proteins: a necessary route to AIDS therapy?
Richter SN, Frasson I, Palù G. Richter SN, et al. Curr Med Chem. 2009;16(3):267-86. doi: 10.2174/092986709787002646. Curr Med Chem. 2009. PMID: 19149577 Review.
Cited by
- Structure-Activity Relationships of Natural and Semisynthetic Plecomacrolides Suggest Distinct Pathways for HIV-1 Immune Evasion and Vacuolar ATPase-Dependent Lysosomal Acidification.
McCauley M, Huston M, Condren AR, Pereira F, Cline J, Yaple-Maresh M, Painter MM, Zimmerman GE, Robertson AW, Carney N, Goodall C, Terry V, Müller R, Sherman DH, Collins KL. McCauley M, et al. J Med Chem. 2024 Mar 28;67(6):4483-4495. doi: 10.1021/acs.jmedchem.3c01574. Epub 2024 Mar 7. J Med Chem. 2024. PMID: 38452116 Free PMC article. - Developments in Exploring Fungal Secondary Metabolites as Antiviral Compounds and Advances in HIV-1 Inhibitor Screening Assays.
Nzimande B, Makhwitine JP, Mkhwanazi NP, Ndlovu SI. Nzimande B, et al. Viruses. 2023 Apr 23;15(5):1039. doi: 10.3390/v15051039. Viruses. 2023. PMID: 37243125 Free PMC article. Review. - Neutron Reflectometry and Molecular Simulations Demonstrate HIV-1 Nef Homodimer Formation on Model Lipid Bilayers.
Heinrich F, Thomas CE, Alvarado JJ, Eells R, Thomas A, Doucet M, Whitlatch KN, Aryal M, Lösche M, Smithgall TE. Heinrich F, et al. J Mol Biol. 2023 Apr 15;435(8):168009. doi: 10.1016/j.jmb.2023.168009. Epub 2023 Feb 10. J Mol Biol. 2023. PMID: 36773691 Free PMC article. - Antiretroviral Drug Discovery Targeting the HIV-1 Nef Virulence Factor.
Emert-Sedlak LA, Shi H, Tice CM, Chen L, Alvarado JJ, Shu ST, Du S, Thomas CE, Wrobel JE, Reitz AB, Smithgall TE. Emert-Sedlak LA, et al. Viruses. 2022 Sep 13;14(9):2025. doi: 10.3390/v14092025. Viruses. 2022. PMID: 36146831 Free PMC article. Review. - The HIV-1 protein Nef activates the Tec family kinase Btk by stabilizing an intermolecular SH3-SH2 domain interaction.
Aryal M, Lin D, Regan K, Du S, Shi H, Alvarado JJ, Ilina TV, Andreotti AH, Smithgall TE. Aryal M, et al. Sci Signal. 2022 Sep 20;15(752):eabn8359. doi: 10.1126/scisignal.abn8359. Epub 2022 Sep 20. Sci Signal. 2022. PMID: 36126115 Free PMC article.
References
- Fackler OT, Baur AS. Live and let die: Nef functions beyond HIV replication. Immunity. 2002;16:493–497. - PubMed
- Joseph AM, Kumar M, Mitra D. Nef: "necessary and enforcing factor" in HIV infection. Curr. HIV. Res. 2005;3:87–94. - PubMed
- Kestler H, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high viral loads and for development of AIDS. Cell. 1991;65:651–662. - PubMed
- Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, McPhee DA, Greenway AL, Ellett A, Chatfield C, Lawson VA, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan JS, Cunningham A, Dwyer D, Dowton D, Mills J. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. - PubMed
- Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 1995;332:228–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA078039/CA/NCI NIH HHS/United States
- U54 MH074411/MH/NIMH NIH HHS/United States
- R01 AI057083-04/AI/NIAID NIH HHS/United States
- R03 MH083223-01/MH/NIMH NIH HHS/United States
- CA78039/CA/NCI NIH HHS/United States
- MH74411/MH/NIMH NIH HHS/United States
- R01 CA081398-09/CA/NCI NIH HHS/United States
- CA81398/CA/NCI NIH HHS/United States
- AI114149/AI/NIAID NIH HHS/United States
- R01 AI057083/AI/NIAID NIH HHS/United States
- R01 CA081398/CA/NCI NIH HHS/United States
- AI57083/AI/NIAID NIH HHS/United States
- R03 MH083223/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Miscellaneous