MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy - PubMed (original) (raw)
MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy
Sara K Lindén et al. PLoS Pathog. 2009 Oct.
Abstract
The bacterium Helicobacter pylori can cause peptic ulcer disease, gastric adenocarcinoma and MALT lymphoma. The cell-surface mucin MUC1 is a large glycoprotein which is highly expressed on the mucosal surface and limits the density of H. pylori in a murine infection model. We now demonstrate that by using the BabA and SabA adhesins, H. pylori bind MUC1 isolated from human gastric cells and MUC1 shed into gastric juice. Both H. pylori carrying these adhesins, and beads coated with MUC1 antibodies, induced shedding of MUC1 from MKN7 human gastric epithelial cells, and shed MUC1 was found bound to H. pylori. Shedding of MUC1 from non-infected cells was not mediated by the known MUC1 sheddases ADAM17 and MMP-14. However, knockdown of MMP-14 partially affected MUC1 release early in infection, whereas ADAM17 had no effect. Thus, it is likely that shedding is mediated both by proteases and by disassociation of the non-covalent interaction between the alpha- and beta-subunits. H. pylori bound more readily to MUC1 depleted cells even when the bacteria lacked the BabA and SabA adhesins, showing that MUC1 inhibits attachment even when bacteria cannot bind to the mucin. Bacteria lacking both the BabA and SabA adhesins caused less apoptosis in MKN7 cells than wild-type bacteria, having a greater effect than deletion of the CagA pathogenicity gene. Deficiency of MUC1/Muc1 resulted in increased epithelial cell apoptosis, both in MKN7 cells in vitro, and in H. pylori infected mice. Thus, MUC1 protects the epithelium from non-MUC1 binding bacteria by inhibiting adhesion to the cell surface by steric hindrance, and from MUC1-binding bacteria by acting as a releasable decoy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
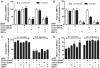
Figure 1. Expression and glycosylation of cell associated and shed MUC1.
(A) Demonstration of MUC1 expression on the apical surface (arrow) of MKN7 gastric cancer cells cultured on a transwell membrane for 3 days post confluency by staining with an anti-MUC1 cytoplasmic domain antibody (CT2). (B–E) Cell associated and shed MUC1 was captured from the high molecular weight fraction of a cellular lysate from MKN7 cells and from three human gastric juice samples (GJ), using a microtitre plate coated with an antibody against the extracellular domain of MUC1 (BC2). Bound MUC1 was detected with another antibody against the extracellular domain of MUC1 (BC3) (B), and the presence of the Leb (C), sialyl-Lea (D) and sialyl-Lex (E) carbohydrate structures on MUC1 were analyzed using antibodies against these structures. Statistics: mean±SEM after subtraction of the mean value of the isotype control coated wells for that sample.
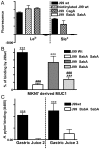
Figure 2. H. pylori binds to synthetic glycoconjugates, cell associated MUC1 and shed MUC1 via the BabA and SabA adhesins.
Adhesion of H. pylori J99 wild type (wt), biotinylated J99wt, a ΔCagA isogenic mutant and a ΔBabASabA isogenic double mutant to FITC labelled Leb or sialyl-Lex conjugated to human serum albumin (A). Cell associated and shed MUC1 were isolated using a microtitre plate coated with the BC2 antibody against the extracellular domain of MUC1 (or isotype control) to capture MUC1 from the high molecular weight fraction of a cellular lysate from the MKN7 gastric cancer cell line (B) and from two human gastric juice samples (C), respectively. Biotinylated J99wt (B, C) or the isogenic adhesion mutants J99ΔBabA (B), J99ΔSabA (B) or a J99ΔBabAΔSabA double mutant (B, C), were added to the wells where MUC1 had been captured by the anti-MUC1 antibody (or isotype control) and binding was detected with streptavidin-HRP. Statistics: mean±SEM after subtraction of the mean value of bacteria-only (A) or isotype control (B-C) coated wells for that sample; ANOVA with Bonferroni post-hoc test (B) or Students t-test (C), N = 6; vs isotype control (B,C ** p<0.01, *** p<0.001;); vs J99wt (B, ### p<0.001).
Figure 3. MUC1 is released from the epithelial surface upon adherence of MUC1-binding beads.
105 fluorescent beads coated with the BC2 antibody against the extracellular domain of MUC1 or an isotype control (IC) were added to 96 well plates with confluent MKN7 epithelial cells and cultured for up to 50 h. Binding was assessed after 4.5, 24 and 50 h by determining fluorescence of washed monolayers (A) and culture supernatants (B). Statistics: Mean ± SEM, n = 9; ***p<0.001 vs 24 h. (C) The amount of MUC1 α-subunit (extracellular domain) in cells treated with anti-MUC1 coated beads compared to isotype control (IC) coated beads was detected by double determinant ELISA using the BC2 (capture) and BC3 (detection) antibodies (***p<0.001). Western blotting (D) was used to detect the MUC1 extracellular domain (using the BC2 antibody) in cell lysates from cells treated with anti-MUC1 coated beads compared to IC for 50 h (top panel, MUC1 has an apparent mobility in the range of 280–440 kDa due to its polydisperse glycosylation). β-actin as a loading/cell density control in the same gel is shown in the middle panel. Concentrated culture supernatants from the same cell monolayers run on a separate SDS-PAGE/Western blot are shown in the lower panel. Origins of the gels are indicated (O).
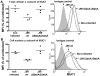
Figure 4. Cell surface MUC1 is depleted during H. pylori infection in vitro.
The concentration of cellular MUC1 from non-infected MKN7 cells and cells co-cultured with H. pylori J99 wild type or J99ΔBabAΔSabA (MOI 1∶1) for 44 h was determined by flow cytometry (with the BC2 antibody) in order to measure the amount of MUC1 per viable cell (the analysis was gated to exclude 7AAD positive dead cells) and distinguish between the total amount of α subunit (the extracellular domain of MUC1) determined in permeabilized cells (A) and the amount of α subunit on the cell surface (B). Representative histograms from the flow cytometry analysis are shown on the right. Median fluorescence intensity (MFI) was determined after subtraction of the MFI using a negative control antibody. Results from 3 independent replicate cultures are shown, expressed as a percentage of the mean MFI of cells from non-infected cultures.
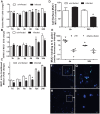
Figure 5. MUC1 is shed from the cell surface and binds H. pylori following co-culture in vitro.
MKN7 cells were uninfected or co-cultured with H. pylori J99 wild type for 1, 2, 4, 6, 12 and 24 h (MOI 10∶1) and total cellular MUC1 determined by BC2/BC3 double determinant ELISA as in Figure 3C (A), MUC1 mRNA concentrations relative to GAPDH were determined by qPCR (B), and concentrations of free MUC1 in conditioned medium determined by ELISA (C). In a further experiment after co-culture of MKN7 cells with H. pylori J99 wild type for 6 and 24 h cell surface MUC1 was assessed in MKN7 cells by flow cytometry as in Figure 4B (D). In a similar experiment both the J99 wild type (MOI 10∶1) and the J99ΔBabAΔSabA (20∶1) were co-cultured with MKN7 cells for 6 and 24 h and the presence of MUC1 on bacteria recovered from the conditioned media was determined by staining with the BC2 antibody or an isotype control followed by flow cytometry (E) and confocal microscopy (F–I). For flow cytometry the MFI of the isotype control was subtracted from the MFI of BC2-stained bacteria for each replicate culture. For confocal microscopy, H. pylori J99 wild type (F, G) or J99ΔBabAΔSabA (H, I) were stained with BC2 (FITC-conjugated secondary antibody, green), DNA (DAPI, blue); scale bars are shown (single scans and additional controls are shown in Figure S2). Scale bars are shown. Statistics: mean±SEM from 4 independent replicate co-cultures; Non-parametric test (Mann Whitney); vs non-infected at same time point (A, C, D) vs J99ΔBabASabA (E), * p<0.05.
Figure 6. Shedding of MUC1 is partially dependent on the MMP-14 protease.
MKN7 cells were transfected with control (scrambled), ADAM17, MMP-14 or ADAM17 plus MMP-14 siRNA. Following 48 h of the transfection, MKN7 cells were infected with H. pylori J99 wild type or cultured uninfected for a further 8 and 24 h. Expression of ADAM17 (A) and MMP-14 (B) mRNA relative to GAPDH was determined by qPCR in uninfected or H. pylori J99 wild type infected cultures (8 h infection, MOI 10∶1). After 8 and 24 h of infection concentrations of cellular MUC1 (C) and free MUC1 in conditioned media (D) were determined by BC2/BC3 double determinant ELISA as in Figure 3C. MUC1 concentrations after infection are presented as a percentage of the mean of the uninfected control for each transfection. Statistics: mean ± SEM from 4 independent replicate co-cultures; Nonparametric test (Mann Whitney); vs scrambled si RNA negative control (* p<0.05).
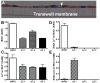
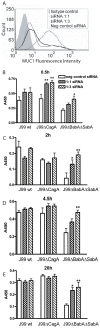
Figure 7. Increased adherence of H. pylori to gastric epithelial cells with reduced Muc1 expression.
(A) Transfection of siRNAs into MKN7 epithelial cells reduced MUC1 expression on the cell surface by 80% with the 1∶1 sequence and 85% with the 1∶3 sequence compared to scrambled siRNA as determined by flow cytometry with an extracellular domain antibody BC2. (B–E) H. pylori J99wt, the CagA deletion mutant J99ΔCagA or adhesion mutant J99ΔBabAΔSabA were co-cultured at a concentration of 8×105 CFU H. pylori/well (MOI 16∶1) in a 96 well plate for 0.5 (B), 2 (C), 4.5 (D) and 20 h (E). Adhesion of H. pylori to cultured cells was determined by ELISA. Data are presented after subtraction of the mean value of the OD for gastric cells without H. pylori. Statistics: Mean±SEM (N = 6); ANOVA, post hoc: Tukey's test; scrambled siRNA vs MUC1 specific siRNA * p<0.05, ** p<0.01.
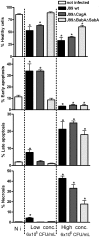
Figure 8. H. pylori induced apoptosis and necrosis is affected by both adhesion and CagA.
MKN7 cells (Not infected; N i, left column) co-cultured with either 6×105 CFU/mL (MOI 1∶1, middle column) or 6×106 CFU/mL (MOI 10∶1, right column) H. pylori (in 500 µl) for 20 h. The graphs show the percentage of healthy cells, cells in early apoptosis (Annexin-V+, 7-AAD−), late apoptosis (Annexin-V+, 7-AAD+ cells) and necrosis (Annexin-V−, 7-AAD+ cells) analyzed by flow cytometry. Statistics: Mean ± SD from 3 independent replicate co-cultures; Mann Whitney U test, vs non-infected * p<0.05.
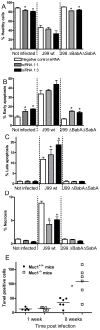
Figure 9. Gastric epithelial cell apoptosis and necrosis is affected by MUC1/Muc1.
(A–D) MKN7 transfected with MUC1 siRNAs 1∶1, 1∶3 (97 and 93% knockdown compared to scrambled siRNA) and scrambled siRNA were co-cultured with 3×105 H. pylori/well (500 µL, MOI 1∶1) for 20 h (A–D). The graphs show (A) the percentage of healthy cells, (B) cells in early apoptosis (Annexin-V+, 7-AAD−), (C) late apoptosis (Annexin-V+, 7-AAD+ cells), and (D) necrosis (Annexin-V−, 7-AAD+ cells), analyzed by flow cytometry. Data were collected for three independent replicate co-cultures and are presented as mean±SD. (E) Six wild-type (Muc1+/+) and Muc1−/− mice were infected with _H. pylori_-SS1 as previously described . The number of TUNEL positive cells per 10 random fields of view in the gastric mucosa was counted at 20× magnification. Statistics: bar = mean, Mann Whitney U test, * p<0.05. Photomicrographs of the TUNEL stained sections are shown in Figure S3.
Similar articles
- Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis.
McGuckin MA, Every AL, Skene CD, Linden SK, Chionh YT, Swierczak A, McAuley J, Harbour S, Kaparakis M, Ferrero R, Sutton P. McGuckin MA, et al. Gastroenterology. 2007 Oct;133(4):1210-8. doi: 10.1053/j.gastro.2007.07.003. Epub 2007 Jul 10. Gastroenterology. 2007. PMID: 17919495 - Helicobacter pylori dwelling on the apical surface of gastrointestinal epithelium damages the mucosal barrier through direct contact.
Zhang C, Zhang H, Yu L, Cao Y. Zhang C, et al. Helicobacter. 2014 Oct;19(5):330-42. doi: 10.1111/hel.12138. Epub 2014 May 14. Helicobacter. 2014. PMID: 24826891 - Fut2-null mice display an altered glycosylation profile and impaired BabA-mediated Helicobacter pylori adhesion to gastric mucosa.
Magalhães A, Gomes J, Ismail MN, Haslam SM, Mendes N, Osório H, David L, Le Pendu J, Haas R, Dell A, Borén T, Reis CA. Magalhães A, et al. Glycobiology. 2009 Dec;19(12):1525-36. doi: 10.1093/glycob/cwp131. Epub 2009 Aug 25. Glycobiology. 2009. PMID: 19706747 Free PMC article. - Helicobacter pylori adhesion to gastric epithelial cells is mediated by glycan receptors.
Magalhães A, Reis CA. Magalhães A, et al. Braz J Med Biol Res. 2010 Jul;43(7):611-8. doi: 10.1590/s0100-879x2010007500049. Epub 2010 Jun 7. Braz J Med Biol Res. 2010. PMID: 20521012 Review. - Adhesion of Helicobacter Species to the Human Gastric Mucosa: A Deep Look Into Glycans Role.
Matos R, Amorim I, Magalhães A, Haesebrouck F, Gärtner F, Reis CA. Matos R, et al. Front Mol Biosci. 2021 May 7;8:656439. doi: 10.3389/fmolb.2021.656439. eCollection 2021. Front Mol Biosci. 2021. PMID: 34026832 Free PMC article. Review.
Cited by
- Targeting the bacteria-host interface: strategies in anti-adhesion therapy.
Krachler AM, Orth K. Krachler AM, et al. Virulence. 2013 May 15;4(4):284-94. doi: 10.4161/viru.24606. Virulence. 2013. PMID: 23799663 Free PMC article. Review. - Assessing Bacterial Interactions Using Carbohydrate-Based Microarrays.
Flannery A, Gerlach JQ, Joshi L, Kilcoyne M. Flannery A, et al. Microarrays (Basel). 2015 Dec 10;4(4):690-713. doi: 10.3390/microarrays4040690. Microarrays (Basel). 2015. PMID: 27600247 Free PMC article. Review. - Role of epithelial mucins during airway infection.
Kim KC. Kim KC. Pulm Pharmacol Ther. 2012 Dec;25(6):415-9. doi: 10.1016/j.pupt.2011.12.003. Epub 2011 Dec 17. Pulm Pharmacol Ther. 2012. PMID: 22198062 Free PMC article. Review. - Mucin O-glycans are natural inhibitors of Candida albicans pathogenicity.
Takagi J, Aoki K, Turner BS, Lamont S, Lehoux S, Kavanaugh N, Gulati M, Valle Arevalo A, Lawrence TJ, Kim CY, Bakshi B, Ishihara M, Nobile CJ, Cummings RD, Wozniak DJ, Tiemeyer M, Hevey R, Ribbeck K. Takagi J, et al. Nat Chem Biol. 2022 Jul;18(7):762-773. doi: 10.1038/s41589-022-01035-1. Epub 2022 Jun 6. Nat Chem Biol. 2022. PMID: 35668191 Free PMC article. - Influence of the viscosity of healthy and diseased human mucins on the motility of Helicobacter pylori.
Su C, Padra M, Constantino MA, Sharba S, Thorell A, Lindén SK, Bansil R. Su C, et al. Sci Rep. 2018 Jun 26;8(1):9710. doi: 10.1038/s41598-018-27732-3. Sci Rep. 2018. PMID: 29946149 Free PMC article.
References
- Targa AC, Cesar AC, Cury PM, Silva AE. Apoptosis in different gastric lesions and gastric cancer: relationship with Helicobacter pylori, overexpression of p53 and aneuploidy. Genet Mol Res. 2007;6:554–565. - PubMed
- Peek RM, Jr, Wirth HP, Moss SF, Yang M, Abdalla AM, et al. Helicobacter pylori alters gastric epithelial cell cycle events and gastrin secretion in Mongolian gerbils. Gastroenterology. 2000;118:48–59. - PubMed
- Handa O, Naito Y, Yoshikawa T. CagA protein of Helicobacter pylori: a hijacker of gastric epithelial cell signaling. Biochem Pharmacol. 2007;73:1697–1702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous