MicroRNA 125a and its regulation of the p53 tumor suppressor gene - PubMed (original) (raw)
MicroRNA 125a and its regulation of the p53 tumor suppressor gene
Yingjie Zhang et al. FEBS Lett. 2009.
Abstract
MicroRNA (miRNA) are a class of non-coding RNA that suppress gene expression by degradation or translational inhibition of target RNA. Several miRNA have been shown to target oncogenes and recently miRNA-125b was shown to translationally and transcriptionally inhibit the p53 gene. Here, we show that an additional isomer of miRNA-125 (miRNA-125a) translationally arrests mRNA of the p53 tumor suppressor gene. The basis of this activity is the high degree of sequence homology between the seed sequence of miR-125a and the 3'-UTR of p53. Our findings add miRNA-125a to the growing list of miRNA with oncogenic targets.
Figures
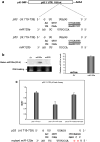
Fig. 1
miR-125 targets the 3′-UTRof p53. (a) The p53-miRNA-125a/b interactome: both isomers of miR-125 (125a, 125b) share homology in their seed sequence (5′ nt 2–7) with the 3′-UTR of p53. (b) Ectopic miRNA-125a expression vector. HEK 293T cells were transfected with miRNA-125a expression vector or control vectors that harbored either irrelevant DNA construct encoding miRNA-378 or no insert (EV). Levels of mature miRNA-125a were visualized by Northern blot (left panel) and quantified by Real Time PCR (right panel). Both assays revealed that wild-type HEK 293T cells express low levels of miRNA-125a that were increased by ∼12-fold upon transfection of miRNA-125a as quantified by Real Time PCR in three independent experiments (results are reported as mean values ±S.D.). (c) Regulation of the p53 3′-UTR by miRNA-125a. Activity of miRNA-125a on the 3′-UTRof p53 was initially assessed by luciferase based reporter assays. The p53 3′-UTR was incorporated into the firefly luciferase gene and run off a single promoter. A constitutively expressed Renilla gene served to normalize transfections. All constructs were introduced into HEK 293T cells with miRNA-125a or an empty control vector (EV) and luminescence was measured at 48 h. miRNA-125a reduced luciferase levels by 40%. The sequence specificity of miRNA-125a/p53 3′-UTR interaction was probed by mutating the seed sequence of miRNA-125a in positions 2 and 4. In comparison to wild-type miRNA-125a, the mutated version had negligible effect (<5%) on the p53 3′-UTR as quantified by levels of luciferase reduction after transfection. Data are presented as mean fold reduction ± S.D. All experiments were performed in triplicate.
Fig. 2
miRNA-125a reduces p53 protein levels by translational arrest of p53 mRNA. (a) Ectopic expression of miRNA-125a reduces p53 protein expression in HEK 293T cells by translational arrest. Cells were transfected with expression vectors for miRNA-125 or an irrelevant construct encoding miRNA-378 and p53 protein levels were quantified by Western blot. GAPDH levels were used to normalize protein input (upper panel). p53 protein reduction by miRNA-125a was not associated with reduction in p53 mRNA levels as quantified by Real Time PCR in cells that had been treated with an empty vector or one encoding miRNA-125a. Data are presented as mean fold reduction ± S.D. All experiments were performed in triplicate. (b) Efficacy of miRNA-125a approaches that of p53 short interfering RNA (siRNA). p53 protein levels were quantified by Western blot in a breast cancer cell line (MCF-7) that had been transfected with either p53 specific siRNA or miRNA-125a. Both agents reduced protein levels of both p53 and downstream protein p21. GAPDH levels were used to normalize protein input. (c) The effect of p53 levels themselves on miRNA-125a was examined in three cell lines. Reduction of p53 protein levels by siRNA had no effect upon levels of endogenously expressed miRNA-125a (upper panel) or miRNA-125b (lower panel) when compared to cells that had been treated with a control empty vector (EV). (d) Several proteins including COP1 and Prih2 are known to negatively regulate p53 protein levels. We found that ectopic expression of miRNA-125a had no effect on levels of either protein, as assessed by Western blot.
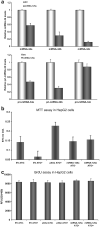
Fig. 3
p53-specific genotypic and phenotypic sequelae of miR-125a expression. (a) p53-specific genotypic sequelae of miRNA-125a expression. miRNA-34a/b/c have been established as transcriptional targets of p53. Levels of all three miRNAs were reduced by 20–80% in cells over expressing miRNA-125a as quantified by Real Time PCR of mature (upper panel) and pri-miRNA levels (lower panel). Data are presented as mean fold reduction ± S.D. compared to cells treated with an empty vector (EV). All experiments were performed in triplicate. (b) p53-specific phenotypic sequelae of miRNA-125a expression. Over-expression of miRNA-125a protects cells from cell death induced by arsenic trioxide. Cells were treated with arsenic trioxide and their viability was quantified by MTT assay. As expected, EV treated cells could not withstand the toxicity of arsenic trioxide. Pre-treatment of cells with siRNA targeting p53 or miRNA-125a preserved viability. All experiments were performed in triplicate and the average and standard deviation of viable cells is shown. (c) The response to arsenic trioxide was assessed by BrdU assay in cells with p53 knockdown or in cells treated with an irrelevant empty vector or one encoding miRNA-125a. Arsenic trioxide or its absence was not related to any significant differences in cellular proliferative capacity in any of the six conditions tested.
Similar articles
- A p53-dependent tumor suppressor network is induced by selective miR-125a-5p inhibition in multiple myeloma cells.
Leotta M, Biamonte L, Raimondi L, Ronchetti D, Di Martino MT, Botta C, Leone E, Pitari MR, Neri A, Giordano A, Tagliaferri P, Tassone P, Amodio N. Leotta M, et al. J Cell Physiol. 2014 Dec;229(12):2106-16. doi: 10.1002/jcp.24669. J Cell Physiol. 2014. PMID: 24819167 - microRNA-1827 represses MDM2 to positively regulate tumor suppressor p53 and suppress tumorigenesis.
Zhang C, Liu J, Tan C, Yue X, Zhao Y, Peng J, Wang X, Laddha SV, Chan CS, Zheng S, Hu W, Feng Z. Zhang C, et al. Oncotarget. 2016 Feb 23;7(8):8783-96. doi: 10.18632/oncotarget.7088. Oncotarget. 2016. PMID: 26840028 Free PMC article. - Regulation of p53 expression and apoptosis by vault RNA2-1-5p in cervical cancer cells.
Kong L, Hao Q, Wang Y, Zhou P, Zou B, Zhang YX. Kong L, et al. Oncotarget. 2015 Sep 29;6(29):28371-88. doi: 10.18632/oncotarget.4948. Oncotarget. 2015. PMID: 26318295 Free PMC article. - Human papillomavirus type 16 E6 induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway.
Au Yeung CL, Tsang TY, Yau PL, Kwok TT. Au Yeung CL, et al. Oncogene. 2011 May 26;30(21):2401-10. doi: 10.1038/onc.2010.613. Epub 2011 Jan 17. Oncogene. 2011. PMID: 21242962 - miR-125a-5p Functions as Tumor Suppressor microRNA And Is a Marker of Locoregional Recurrence And Poor prognosis in Head And Neck Cancer.
Vo DT, Karanam NK, Ding L, Saha D, Yordy JS, Giri U, Heymach JV, Story MD. Vo DT, et al. Neoplasia. 2019 Sep;21(9):849-862. doi: 10.1016/j.neo.2019.06.004. Epub 2019 Jul 18. Neoplasia. 2019. PMID: 31325708 Free PMC article.
Cited by
- Hepatitis B virus and microRNAs: Complex interactions affecting hepatitis B virus replication and hepatitis B virus-associated diseases.
Lamontagne J, Steel LF, Bouchard MJ. Lamontagne J, et al. World J Gastroenterol. 2015 Jun 28;21(24):7375-99. doi: 10.3748/wjg.v21.i24.7375. World J Gastroenterol. 2015. PMID: 26139985 Free PMC article. Review. - Biogenesis, evolution and functional targets of microRNA-125a.
Potenza N, Russo A. Potenza N, et al. Mol Genet Genomics. 2013 Sep;288(9):381-9. doi: 10.1007/s00438-013-0757-5. Epub 2013 Jun 20. Mol Genet Genomics. 2013. PMID: 23783428 Review. - miR-125a Suppresses TrxR1 Expression and Is Involved in H2O2-Induced Oxidative Stress in Endothelial Cells.
Chen F, Liu H, Wu J, Zhao Y. Chen F, et al. J Immunol Res. 2018 Aug 26;2018:6140320. doi: 10.1155/2018/6140320. eCollection 2018. J Immunol Res. 2018. PMID: 30225271 Free PMC article. - Genetic subclonal complexity and miR125a-5p down-regulation identify a subset of patients with inferior outcome in low-risk CLL patients.
Rigolin GM, Saccenti E, Rizzotto L, Ferracin M, Martinelli S, Formigaro L, Cibien F, Cavallari M, Lista E, Daghia G, Sofritti O, Ciccone M, Cavazzini F, Lupini L, Bassi C, Zagatti B, Negrini M, Cuneo A. Rigolin GM, et al. Oncotarget. 2014 Jan 15;5(1):140-9. doi: 10.18632/oncotarget.1382. Oncotarget. 2014. PMID: 24334759 Free PMC article. - Tumor suppressor protein Pdcd4 inhibits translation of p53 mRNA.
Wedeken L, Singh P, Klempnauer KH. Wedeken L, et al. J Biol Chem. 2011 Dec 16;286(50):42855-62. doi: 10.1074/jbc.M111.269456. Epub 2011 Oct 27. J Biol Chem. 2011. PMID: 22033922 Free PMC article.
References
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. - PubMed
- Esquela-Kerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. - PubMed
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. - PubMed
- Bentwich I, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37(7):766–770. - PubMed
- Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431(7006):343–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DA013911/DA/NIDA NIH HHS/United States
- P20 RR017695/RR/NCRR NIH HHS/United States
- P30 AI042853/AI/NIAID NIH HHS/United States
- P20 RR025179/RR/NCRR NIH HHS/United States
- 1P20RR025179/RR/NCRR NIH HHS/United States
- P20 GM103421/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous