Safety, tolerability, pharmacokinetics and pharmacodynamics of the oral cyclin-dependent kinase inhibitor AZD5438 when administered at intermittent and continuous dosing schedules in patients with advanced solid tumours - PubMed (original) (raw)
Clinical Trial
. 2010 Apr;21(4):884-894.
doi: 10.1093/annonc/mdp377. Epub 2009 Oct 13.
G K Schwartz 2, M R Middleton 3, D D Amakye 4, H Swaisland 4, R S Midgley 3, M Ranson 5, S Danson 6, H Calvert 7, R Plummer 7, C Morris 4, R D Carvajal 2, L R Chirieac 8, J H M Schellens 9, G I Shapiro 10
Affiliations
- PMID: 19825886
- PMCID: PMC2844945
- DOI: 10.1093/annonc/mdp377
Clinical Trial
Safety, tolerability, pharmacokinetics and pharmacodynamics of the oral cyclin-dependent kinase inhibitor AZD5438 when administered at intermittent and continuous dosing schedules in patients with advanced solid tumours
D S Boss et al. Ann Oncol. 2010 Apr.
Abstract
Background: AZD5438 is an orally bioavailable inhibitor of cyclin E-cdk2, cyclin A-cdk2 and cyclin B-cdk1 complexes. Three phase I studies assessed the clinical safety, tolerability, pharmacokinetics and pharmacodynamics of AZD5438 when administered in different dosing schedules.
Patients and methods: AZD5438 was administered four times daily, once every 7 days (study 1), for 14 consecutive days followed by 7 days of rest (study 2), or continuously (study 3), to patients with advanced solid tumours. Dose escalation proceeded until the emergence of dose-limiting toxic effects.
Results: Sixty-four patients were included across the three studies (19, 17 and 28, respectively). Nausea and vomiting were the most common adverse events. When dosed continuously, 40 mg four times daily was considered intolerable, and due to safety issues, all studies were terminated prematurely. Consequently, no intolerable dose was identified during the weekly schedule. Pharmacokinetics demonstrated dose-proportional exposure, high interpatient variability and accumulation after multiple doses. Skin biopsies indicated reduced retinoblastoma protein phosphorylation at cdk2 phospho-sites; other pharmacodynamic assessments did not reveal consistent trends.
Conclusions: AZD5438 was generally well tolerated in a weekly dosing schedule, but not in continuous schedules. The clinical development programme for AZD5438 was discontinued owing to tolerability and exposure data from these studies.
Figures
Figure 1.
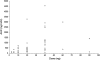
Exposure [area under the curve (AUC)] versus AZD5438 dose. This figure shows the AUC as a function of AZD5438 dose. The AUCs were calculated after a single dose of AZD5438, on day 1 of treatment. There is a dose-proportional increase in exposure, but a high interpatient variability in exposure to AZD5438.
Figure 2.
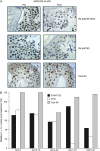
Total and phospho-Rb staining in skin biopsies. (A) Skin biopsies were obtained pre-treatment (pre) and 2 h after the first dose on day 22 of continuous ADZ5438 dosing. Five-micrometer sections from formalin-fixed, paraffin-embedded samples were subjected to immunohistochemistry with the indicated antibodies. Results from patient 23 are shown (treated at the 40-mg dose level), demonstrating reduced Rb staining at the S249/T252 and S780 phospho-sites in the keratinocyte layers, suggestive of reduced cyclin-dependent kinase activity after treatment. Total Rb staining is maintained in the post-treatment sample. (B) Immunohistochemical data were quantified by scoring the nuclei of 100–200 keratinocytes as 0, 1+ or 2+. The percentage of positive nuclei was considered as 100 for each pre-treatment sample. Bars indicate the percent positive staining (1+, 2+) in each post-treatment sample, relative to the pre-treatment sample. The _x_-axis designations indicate the patient number and the dose of AZD5438 administered. In these samples, reductions in phospho-Rb staining were noted, while total Rb staining was maintained after treatment.
Figure 3.
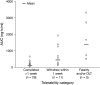
Post hoc analysis: exposure to AZD5438 and tolerability outcome in continuous dosing studies. Owing to the lack of multiple-dose data for many of the patients, the total daily area under the curve was calculated from day 1 pharmacokinetic measurements. Tolerability categories were (1) patients who completed at least the initial safety assessment period (21 or 28 days) or withdrew after 1 week due to disease progression; (2) patients who withdrew within 1 week of starting treatment due to poor tolerability or for disease-related reasons and (3) patients who discontinued due to fatality and/or dose-limiting toxicity.
Similar articles
- A phase l study of three different dosing schedules of the oral aurora kinase inhibitor MSC1992371A in patients with solid tumors.
Mita M, Gordon M, Rejeb N, Gianella-Borradori A, Jego V, Mita A, Sarantopoulos J, Sankhala K, Mendelson D. Mita M, et al. Target Oncol. 2014 Sep;9(3):215-24. doi: 10.1007/s11523-013-0288-3. Epub 2013 Jul 6. Target Oncol. 2014. PMID: 23832397 Clinical Trial. - Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial.
Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, DeMarini DJ, Cox DS, Xu Y, Morris SR, Peddareddigari VG, Le NT, Hart L, Bendell JC, Eckhardt G, Kurzrock R, Flaherty K, Burris HA 3rd, Messersmith WA. Infante JR, et al. Lancet Oncol. 2012 Aug;13(8):773-81. doi: 10.1016/S1470-2045(12)70270-X. Epub 2012 Jul 16. Lancet Oncol. 2012. PMID: 22805291 Clinical Trial. - Phase I dose-escalation studies of roniciclib, a pan-cyclin-dependent kinase inhibitor, in advanced malignancies.
Bahleda R, Grilley-Olson JE, Govindan R, Barlesi F, Greillier L, Perol M, Ray-Coquard I, Strumberg D, Schultheis B, Dy GK, Zalcman G, Weiss GJ, Walter AO, Kornacker M, Rajagopalan P, Henderson D, Nogai H, Ocker M, Soria JC. Bahleda R, et al. Br J Cancer. 2017 Jun 6;116(12):1505-1512. doi: 10.1038/bjc.2017.92. Epub 2017 May 2. Br J Cancer. 2017. PMID: 28463960 Free PMC article. Clinical Trial. - Trilaciclib: First Approval.
Dhillon S. Dhillon S. Drugs. 2021 May;81(7):867-874. doi: 10.1007/s40265-021-01508-y. Drugs. 2021. PMID: 33861388 Review. - The rationale of dose-response curves in selecting cancer drug dosing.
Martin JH, Dimmitt S. Martin JH, et al. Br J Clin Pharmacol. 2019 Oct;85(10):2198-2204. doi: 10.1111/bcp.13979. Epub 2019 Jun 20. Br J Clin Pharmacol. 2019. PMID: 31077412 Free PMC article. Review.
Cited by
- AZD5438, an inhibitor of Cdk1, 2, and 9, enhances the radiosensitivity of non-small cell lung carcinoma cells.
Raghavan P, Tumati V, Yu L, Chan N, Tomimatsu N, Burma S, Bristow RG, Saha D. Raghavan P, et al. Int J Radiat Oncol Biol Phys. 2012 Nov 15;84(4):e507-14. doi: 10.1016/j.ijrobp.2012.05.035. Epub 2012 Jul 12. Int J Radiat Oncol Biol Phys. 2012. PMID: 22795803 Free PMC article. - Cyclin-dependent kinases (cdks) and the DNA damage response: rationale for cdk inhibitor-chemotherapy combinations as an anticancer strategy for solid tumors.
Johnson N, Shapiro GI. Johnson N, et al. Expert Opin Ther Targets. 2010 Nov;14(11):1199-212. doi: 10.1517/14728222.2010.525221. Expert Opin Ther Targets. 2010. PMID: 20932174 Free PMC article. Review. - Double-strand DNA break repair: molecular mechanisms and therapeutic targets.
Tan J, Sun X, Zhao H, Guan H, Gao S, Zhou PK. Tan J, et al. MedComm (2020). 2023 Oct 5;4(5):e388. doi: 10.1002/mco2.388. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37808268 Free PMC article. Review. - Adapalene inhibits the activity of cyclin-dependent kinase 2 in colorectal carcinoma.
Shi XN, Li H, Yao H, Liu X, Li L, Leung KS, Kung HF, Lin MC. Shi XN, et al. Mol Med Rep. 2015 Nov;12(5):6501-8. doi: 10.3892/mmr.2015.4310. Epub 2015 Sep 10. Mol Med Rep. 2015. PMID: 26398439 Free PMC article. - Mitochondrial dysfunction RAD51, and Ku80 proteolysis promote apoptotic effects of Dinaciclib in Bcl-xL silenced cells.
Premkumar DR, Jane EP, Thambireddy S, Sutera PA, Cavaleri JM, Pollack IF. Premkumar DR, et al. Mol Carcinog. 2018 Apr;57(4):469-482. doi: 10.1002/mc.22771. Epub 2017 Dec 30. Mol Carcinog. 2018. PMID: 29240261 Free PMC article.
References
- Reed SI. Control of the G1/S transition. Cancer Surv. 1997;29:7–23. - PubMed
- Harbour JW, Luo RX, Dei Santi A, et al. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. - PubMed
- Pines J. The cell cycle kinases. Semin Cancer Biol. 1994;5:305–313. - PubMed
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous