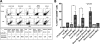CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization - PubMed (original) (raw)
CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization
Hernan Roca et al. J Biol Chem. 2009.
Abstract
CCL2 and interleukin (IL)-6 are among the most prevalent cytokines in the tumor microenvironment, with expression generally correlating with tumor progression and metastasis. CCL2 and IL-6 induced expression of each other in CD11b(+) cells isolated from human peripheral blood. It was demonstrated that both cytokines induce up-regulation of the antiapoptotic proteins cFLIP(L) (cellular caspase-8 (FLICE)-like inhibitory protein), Bcl-2, and Bcl-X(L) and inhibit the cleavage of caspase-8 and subsequent activation of the caspase-cascade, thus protecting cells from apoptosis under serum deprivation stress. Furthermore, both cytokines induced hyperactivation of autophagy in these cells. Upon CCL2 or IL-6 stimulation, CD11b(+) cells demonstrated a significant increase in the mannose receptor (CD206) and the CD14(+)/CD206(+) double-positive cells, suggesting a polarization of macrophages toward the CD206(+) M2-type phenotype. Caspase-8 inhibitors mimicked the cytokine-induced up-regulation of autophagy and M2 polarization. Furthermore, E64D and leupeptin, which are able to function as inhibitors of autophagic degradation, reversed the effect of caspase-8 inhibitors in the M2-macrophage polarization, indicating a role of autophagy in this mechanism. Additionally, in patients with advanced castrate-resistant prostate cancer, metastatic lesions exhibited an increased CD14(+)/CD206(+) double-positive cell population compared with normal tissues. Altogether, these findings suggest a role for CCL2 and IL-6 in the survival of myeloid monocytes recruited to the tumor microenvironment and their differentiation toward tumor-promoting M2-type macrophages via inhibition of caspase-8 cleavage and enhanced autophagy.
Figures
FIGURE 1.
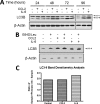
CCL2 and IL-6 exhibit mutual induction in human CD11b+ cells. A and B, isolated CD11b+ cells were plated in serum-free RPMI and allowed to attach at 37 °C prior to treatment. Following attachment, the cells were treated or not with 100 ng/ml recombinant CCL2 or IL-6 for 48 h. The chemokine expression profiles were evaluated in cell supernatants by using inflammation array 3 and cytokine array 5 (RayBiotech, Inc.) as indicated. The arrays displayed are representative examples of three assays. C, bar graph depicts densitometric analysis of all spots corresponding to CCL2 and IL-6, from both inflammation array 3 and cytokine array 5. The data presented represents average CCL2 and IL-6 spot density values (from n = 3 arrays) in response to treatment with IL-6 or CCL2, respectively. The data were normalized to array-specific positive controls and then compared with untreated cells to establish average fold changes.
FIGURE 2.
CCL2 and IL-6 protect CD11b+ myeloid cells from apoptotic death. Isolated CD11b+ cells were plated in either 96- or 6-well plates at a density of 1.5 × 106 cells/ml in serum-free RPMI. The cells were then treated or not with 100 ng/ml CCL2 or IL-6. A, CD11b+ cell survival in response to CCL2 or IL-6 treatment. Survival was evaluated by WST-1 dye conversion immediately preceding cytokine treatment and at 24-h increments, up to 72 h, following stimulation. The data for each treatment condition is representative of n = 10 samples and is normalized to average, 0-h absorbance. B, cell morphology analyzed 48 h post-treatment with the indicated cytokines. The images were obtained using an Olympus IX71 microscope (20× objective), fitted with an Olympus DP71 camera. C, total percentages of apoptotic cells 48 and 96 h post-chemokine treatment, as evaluated by flow cytometry. Sub-G0/G1 content (apoptotic cells, denoted as M2) was assessed by PI fluorescence and was determined by FSC/SSC gating. D, protein lysates were collected in 24-h increments to evaluate the expression of activated/cleaved caspases and lamin A, as well as anti-apoptotic proteins (Bcl-2 and Bcl-XL) by Western blot. E, CCL2 and IL-6 effectively induce the caspase-8 inhibitor cFLIPL and prevent caspase-8 cleavage, as determined by Western blot 48 h post-treatment. The antibody against cleaved caspase-8 recognizes cleavage products p43/p41 and p18; all of the bands are shown here (separated by a single black line) from the same gel with the empty region in between the bands, corresponding to the cleavage products, removed. β-Actin was evaluated as a loading control for all blots.
FIGURE 3.
CCL2 and IL-6 hyperactivate autophagy in human CD11b+ cells. A, isolated human CD11b+ cells were treated or not with 100 ng/ml CCL2 or IL-6. Protein lysates were collected at 24-h increments, up to 96 h, and conversion of LC3-I to LC3-II was evaluated by Western blot. Both LC3-I (upper band; 16 kDa) and its phosphatidylethanolamine-conjugated form, LC3-II (lower band; 14 kDa) are detected by the LC3B antibody. B, because LC3-II itself is degraded by autophagy, a subset of the chemokine-stimulated CD11b+ cells were also treated with protease inhibitors leupeptin and E64D at 10 μg/ml each. The cells were then incubated for an additional 48 h at 37 °C, and the protein lysates were collected (t = 72 h) and analyzed by Western blot, using the LC3B antibody. C, densitometric analysis of LC3-II bands obtained from the Western blot (Fig. 3_B_) of CD11b+ for control, CCL2, and IL-6 ± protease inhibitors. The data displayed in the bar graph represents the spot density fold change in response to protease inhibitors, for each treatment group, and is normalized to corresponding β-actin blot densities.
FIGURE 4.
Protection from apoptosis by IL-6 is partially dependent upon the induction of CCL2. Isolated human CD11b+ cells were plated and treated or not with CCL2 ± IL-6 neutralizing antibody (_IL-6_α) or IL-6 ± CCL2 neutralizing Ab (_CCL2_α). 48 h post-treatment, the protein lysates were collected, and the expression of activated/cleaved caspases, PARP, and lamin A (A) and Bcl-2, Bcl-XL, and LC3 (B) were evaluated by Western blot. β-Actin was assessed as a loading control for all blots. C, CD11b+ cell morphology, analyzed 48 h post-cytokine ± neutralizing antibody treatment. The images were obtained using an Olympus IX71 microscope (20× objective) fitted with an Olympus DP71 camera.
FIGURE 5.
CCL2 and IL-6 induce CD11b+-monocyte differentiation toward the M2-type macrophage phenotype. Characterization of cell populations from human CD11b+ peripheral blood mononuclear cells (PBMCs) was performed by flow cytometry, using the FITC-conjugated CD14 antibody and the PE-conjugated CD206 (mannose receptor) antibody. Flow cytometry analysis was performed immediately following CD11b-magnetic bead isolation to detect receptors levels in the undifferentiated peripheral blood mononuclear cells (A) and 48 h post-treatment with CCL2 or IL-6 (B–D), compared with untreated (control) cells. Cell populations were gated into regions R1, R2, and R3 based on FSC/SSC parameters. CD14+ cells are shown in the lower right quadrants, CD206+ cells are shown in the upper left quadrants, and CD14+/CD206+ double-positive cells are shown in the upper right quadrants. The data displayed are representative examples of n = 3 experiments with identical treatment and cell isolation parameters. E, graphic depiction of compiled percentages (n = 3; all gates included) of cells that express the respective cell surface receptors for: CD11b+ peripheral blood mononuclear cells (immediately after isolation) and CCL2/IL-6-treated and control (untreated) CD11b+ cells, as determined by flow cytometry.
FIGURE 6.
Caspase-8 inhibitor-I, IETD-CHO, increases autophagic activity and promotes M2-type macrophage polarization. A and B, isolated CD14+ monocytes were plated and treated or not with 2.5 and 5 μ
m
caspase 8 inhibitor-I, IETD-CHO, or respective volumes of Me2SO for 48 h. A, the effect of the inhibition of caspase-8 cleavage on autophagy was evaluated by LC3 immunodetection. B, the increase in autophagy observed in Fig. 6_A_ by caspase-8 inhibitor-I was validated by treatment with protease inhibitors E64D and leupeptin. Collected protein lysates were analyzed by Western blot to evaluate full-length caspase-8 (p55 and p53, the two predominant isoforms are indicated) and LC3 conversion. β-Actin was assessed as a loading control for all blots. C–F, isolated CD14+ monocytes were plated in the presence and absence of caspase-8 inhibitor-I (2.5 μ
m
). The cells were incubated at 37 °C for 16 h and then treated or not with E64D and leupeptin (inhibitors of autophagic degradation, 10 μg/ml each). 48 h post-treatment, the cells were gently scraped and subjected to flow cytometry analysis with: CD14 FITC-conjugated antibodies, CD206 PE-conjugated antibodies, or a combination of both. Dot plots shown are representative of observed data from n = 2 replicate experiments. The cell populations were gated into regions R1, R2, and R3, based on FSC/SSC parameters. CD14+ cells are shown in the lower-right quadrants, CD206+ cells are shown in the upper left quadrants, and CD14+/CD206+ double-positive cells are shown in the upper right quadrants. The percentages of cell populations, by region, are displayed in the tables (left side of each image) for all conditions. G, table summarizing total percentage of cell populations (from all gates), as determined by flow cytometry.
FIGURE 7.
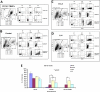
CD14+/CD206+ double-positive cell populations are enhanced in human prostate cancer metastatic lesions. The cells from tumor and respective normal tissues were collected from three patients, corresponding to University of Michigan rapid autopsy numbers 53–55. A, representative flow cytometry analysis showing the percentage of total cell populations that were CD14+/CD206+ double-positive using FITC-conjugated CD14 and PE-conjugated CD206 antibodies. The cells that express both CD14 and CD206 surface receptors are displayed in the upper right quadrants of the dot plots. The table summarizes the percentage of total double-positive cells for all tumor (right) and respective normal (left) tissue samples. B, graphic representation of a compiled data analysis from rapid autopsy tissues.
FIGURE 8.
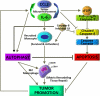
Proposed mechanism by which CCL2 and IL-6 potentiate tumor progression by protecting tumor infiltrating monocytes and inducing their differentiation toward M2-type macrophages. CCL2 and IL-6 induce each other and boost their expression in the tumor microenvironment. Both cytokines inhibit the apoptotic cleavage of caspase-8 and promote enhanced autophagic activity to protect the monocytes recruited to the tumor and, at the same time, induce their differentiation toward M2-type macrophages.
Similar articles
- [Adipose-derived stem cells promote the polarization from M1 macrophages to M2 macrophages].
Yin X, Pang C, Bai L, Zhang Y, Geng L. Yin X, et al. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016 Mar;32(3):332-8. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016. PMID: 26927552 Chinese. - CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis.
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. Qian BZ, et al. Nature. 2011 Jun 8;475(7355):222-5. doi: 10.1038/nature10138. Nature. 2011. PMID: 21654748 Free PMC article. - Notch signaling triggered via the ligand DLL4 impedes M2 macrophage differentiation and promotes their apoptosis.
Pagie S, Gérard N, Charreau B. Pagie S, et al. Cell Commun Signal. 2018 Jan 10;16(1):4. doi: 10.1186/s12964-017-0214-x. Cell Commun Signal. 2018. PMID: 29321062 Free PMC article. - Triggering of protease-activated receptors (PARs) induces alternative M2 macrophage polarization with impaired plasticity.
García-González G, Sánchez-González A, Hernández-Bello R, González GM, Franco-Molina MA, Coronado-Cerda EE, Palma-Nicolás JP. García-González G, et al. Mol Immunol. 2019 Oct;114:278-288. doi: 10.1016/j.molimm.2019.08.004. Epub 2019 Aug 13. Mol Immunol. 2019. PMID: 31419704 - Effect of colorectal cancer-derived extracellular vesicles on the immunophenotype and cytokine secretion profile of monocytes and macrophages.
Popēna I, Ābols A, Saulīte L, Pleiko K, Zandberga E, Jēkabsons K, Endzeliņš E, Llorente A, Linē A, Riekstiņa U. Popēna I, et al. Cell Commun Signal. 2018 Apr 24;16(1):17. doi: 10.1186/s12964-018-0229-y. Cell Commun Signal. 2018. PMID: 29690889 Free PMC article.
Cited by
- Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis.
Lippitz BE, Harris RA. Lippitz BE, et al. Oncoimmunology. 2016 May 11;5(5):e1093722. doi: 10.1080/2162402X.2015.1093722. eCollection 2016 May. Oncoimmunology. 2016. PMID: 27467926 Free PMC article. Review. - Therapeutic Approaches Targeting the Natural Killer-Myeloid Cell Axis in the Tumor Microenvironment.
Carnevalli LS, Ghadially H, Barry ST. Carnevalli LS, et al. Front Immunol. 2021 Apr 19;12:633685. doi: 10.3389/fimmu.2021.633685. eCollection 2021. Front Immunol. 2021. PMID: 33953710 Free PMC article. Review. - Current Status and Future Perspectives of Checkpoint Inhibitor Immunotherapy for Prostate Cancer: A Comprehensive Review.
Kim TJ, Koo KC. Kim TJ, et al. Int J Mol Sci. 2020 Jul 31;21(15):5484. doi: 10.3390/ijms21155484. Int J Mol Sci. 2020. PMID: 32751945 Free PMC article. Review. - Senescent skeletal muscle fibroadipogenic progenitors recruit and promote M2 polarization of macrophages.
Zhang X, Ng YE, Chini LCS, Heeren AA, White TA, Li H, Huang H, Doolittle ML, Khosla S, LeBrasseur NK. Zhang X, et al. Aging Cell. 2024 Mar;23(3):e14069. doi: 10.1111/acel.14069. Epub 2023 Dec 19. Aging Cell. 2024. PMID: 38115574 Free PMC article. - IL-6 in human cytomegalovirus secretome promotes angiogenesis and survival of endothelial cells through the stimulation of survivin.
Botto S, Streblow DN, DeFilippis V, White L, Kreklywich CN, Smith PP, Caposio P. Botto S, et al. Blood. 2011 Jan 6;117(1):352-61. doi: 10.1182/blood-2010-06-291245. Epub 2010 Oct 7. Blood. 2011. PMID: 20930069 Free PMC article.
References
- Balkwill F., Mantovani A. (2001) Lancet 357, 539–545 - PubMed
- Bingle L., Brown N. J., Lewis C. E. (2002) J. Pathol. 196, 254–265 - PubMed
- Pollard J. W. (2004) Nat. Rev. Cancer 4, 71–78 - PubMed
- Brigati C., Noonan D. M., Albini A., Benelli R. (2002) Clin. Exp. Metastasis 19, 247–258 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA 46592/CA/NCI NIH HHS/United States
- P50 CA69568/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- CA093900/CA/NCI NIH HHS/United States
- P50 CA069568/CA/NCI NIH HHS/United States
- P01 CA093900/CA/NCI NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials