Non-muscle myosin II takes centre stage in cell adhesion and migration - PubMed (original) (raw)
Review
Non-muscle myosin II takes centre stage in cell adhesion and migration
Miguel Vicente-Manzanares et al. Nat Rev Mol Cell Biol. 2009 Nov.
Abstract
Non-muscle myosin II (NM II) is an actin-binding protein that has actin cross-linking and contractile properties and is regulated by the phosphorylation of its light and heavy chains. The three mammalian NM II isoforms have both overlapping and unique properties. Owing to its position downstream of convergent signalling pathways, NM II is central in the control of cell adhesion, cell migration and tissue architecture. Recent insight into the role of NM II in these processes has been gained from loss-of-function and mutant approaches, methods that quantitatively measure actin and adhesion dynamics and the discovery of NM II mutations that cause monogenic diseases.
Figures
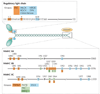
Figure 1. Domain structure of NM II
a | The subunit and domain structure of non-muscle myosin II (NM II), which forms a dimer through interactions between the α-helical coiled-coil rod domains. The globular head domain contains the actin-binding regions and the enzymatic Mg2+-ATPase motor domains. The essential light chains (ELCs) and the regulatory light chains (RLCs) bind to the heavy chains at the lever arms that link the head and rod domains. In the absence of RLC phosphorylation, NM II forms a compact molecule through a head to tail interaction. This results in an assembly-incompetent form (10S; left) that is unable to associate with other NM II dimers. On RLC phosphorylation, the 10S structure unfolds and becomes an assembly-competent form (6S). S-1 is a fragment of NM II that contains the motor domain and neck but lacks the rod domain and is unable to dimerize. Heavy meromyosin (HMM) is a fragment that contains the motor domain, neck and enough of the rod to effect dimerization. b | NM II molecules assemble into bipolar filaments through interactions between their rod domains. These filaments bind to actin through their head domains and the ATPase activity of the head enables a conformational change that moves actin filaments in an anti-parallel manner. Bipolar myosin filaments link actin filaments together in thick bundles that form cellular structures such as stress fibres.
Figure 2. Regulation of NM II activation and filament formation
Multiple kinases, including myosin light chain kinase (MLCK; also known as MYLK), Rho-associated, coiled coil-containing kinase (ROCK), citron kinase, myotonic dystrophy kinase-related CDC42-binding kinase (MRCK; also known as CDC42BP) and leucine zipper interacting kinase (ZIPK; also known as DAPK3) can phosphorylate the regulatory light chain (RLC) of non-muscle myosin II (NM II) on Ser19 or on Thr18 and Ser19 to activate it. Protein kinase C (PKC) can phosphorylate the RLC on Ser1, Ser2 and Thr9 to inhibit NM II. Human RLC is encoded by myosin light chain 9 (
MYL9
). In addition to RLC phosphorylation, NM II filament assembly is regulated by phosphorylation of the NM II heavy chain (NMHC II) coiled-coil and tail domains. The phosphorylation sites and the corresponding kinases, including transient receptor potential melastatin 7 (TRPM7), PKC proteins and casein kinase II (CK II), are shown for human NMHC IIA, NMHC IIB and NMHC IIC. Phosphorylation of NMHC II by CK II blocks the binding of S100A4 (also known as MTS1) to the tail of NM II to prevent it from inhibiting myosin filament assembly. Human NMHC IIA is encoded by myosin heavy chain 9 (MYH9), NMHC IIB is encoded by MYH10 and NMHC IIC is encoded by MYH14. Note that each phosphorylation site and the kinase that phosphorylates it are depicted in the same colour.
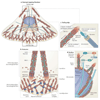
Figure 3. Multiple roles of NM II in cell migration
a | A polarized, migrating fibroblast. Areas of the cell in which non-muscle myosin II (NM II) has an active role are boxed and expanded in parts b– d. b | NM II regulates retrograde flow in the lamellum and promotes adhesion maturation, thereby limiting protrusion. Nascent adhesions form in the lamellipodium, in which dendritic actin branching mediated by the Arp2/3 complex also occurs. At the lamellipodium–lamellum interface, actin is depolymerized or bundled and adhesions disassemble or mature. A schematic of adhesions maturing in the lamellum is also shown. NM II localizes to actin bundles contacting growing adhesions, forming a striated pattern with α-actinin. In other cells, such as in neuronal growth cones, NM II may have a more direct role controlling retrograde flow in the peripheral zone. c | NM II participates in adhesion disassembly at the rear of the cell. NM IIA-mediated contraction, calpain-dependent cleavage of adhesion components and microtubule targeting coordinately induce adhesion disassembly. d | NM II has a role in nuclear positioning and orienting the microtubule-organizing centre (MTOC) and Golgi, which are important for cell polarization. NM II is thought to act in concert with the CDC42–partitioning defective 3 (PAR3) or PAR6–protein kinase Cζ (PKCζ) –glycogen synthase kinase 3 (GSK3) pathway to polarize the cell. Myotonic dystrophy kinase-related CDC42-binding kinase (MRCK; also known as CDC42BP) activates NM II and regulates its effect on nucleus repositioning. APC, adenomatous polyposis coli; DIAPH1, diaphanous 1; EB1, end binding protein 1 (also known as MAPRE1).
Figure 4. NM II in integrin-mediated adhesion
Integrins that are bound to the extracellular matrix (ECM) are linked to the actin cytoskeleton through an actin linkage that is formed by multiple molecules, including talin, vinculin and α-actinin. Kinases such as focal adhesion kinase (FAK) and Src, and adaptors such as paxillin, are also recruited and trigger the downstream activation of Rho GTPases such as Rac through adaptor and activating proteins. Representative pathways and associations are shown, including the activation of Rac through paxillin by the CRK-associated substrate (p130CAS; also known as BCAR1)–CRK–dedicator of cytokinesis 1 (DOCK1; also known as DOCK180) and G protein-coupled receptor kinase interacting ArfGAP (GIT)–β−Pix (also known as ARHGEF7) pathways. Activated Rac induces actin polymerization through the Arp2/3 complex, which can also interact with some of the molecules of the actin linkage, such as vinculin and FAK. Rac is also thought to locally inhibit NM II activation. The activation of RhoGEFs by integrins, and the subsequent activation of RHOA and Rho-associated, coiled coil-containing kinase (ROCK), activates NM II. ROCK activates NM II directly by phosphorylating the regulatory light chains (RLCs) or by inactivating myosin phosphatase, which in turn promotes RLC dephosphorylation. The pathways are spatially and temporally regulated. Additionally, the activation and inactivation of NM II itself affects adhesive signalling by triggering conformational changes in the mechanoresponsive molecules shown (pink boxes), which induces the clustering of the indicated adhesion proteins (blue boxes) by reinforcing or weakening the linkage of the adhesion and the actin cytoskeleton. AM, adaptor module; MYPT1, myosin phosphatase-targeting subunit 1 (also known as PPP1R12A); PP1, protein phosphatase 1.
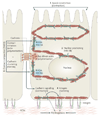
Figure 5. Roles of NM II in epithelial cell polarization
The different roles of non-muscle myosin II (NM II) in epithelial cell polarization. NM II is involved in apical constriction (step 1), which leads to important morphogenic movements such as dorsal closure (closure of the epidermis over the amnioserosa during embryogenesis) in Drosophila melanogaster. In addition, NM II regulates nuclear positioning (step 2), in a similar manner to how it does this in fibroblasts (see FIG 3). NM II and RHOA signalling also stabilize cell–cell contacts by reinforcing them through actin cross-linking (known as contact compaction; step 3). The initial contacts are formed as a result of Rac-driven actin polymerization, but NM IIA is required for contact formation and reinforcement and cadherin clustering. NM II also mediates crosstalk between homophilic cadherin contact-initiated signalling and extracellular matrix (ECM) remodelling triggered by integrin activation and clustering (step 4).
Similar articles
- Myosin phosphatase targeting subunit 1 affects cell migration by regulating myosin phosphorylation and actin assembly.
Xia D, Stull JT, Kamm KE. Xia D, et al. Exp Cell Res. 2005 Apr 1;304(2):506-17. doi: 10.1016/j.yexcr.2004.11.025. Epub 2004 Dec 30. Exp Cell Res. 2005. PMID: 15748895 - Myosin II in mechanotransduction: master and commander of cell migration, morphogenesis, and cancer.
Aguilar-Cuenca R, Juanes-García A, Vicente-Manzanares M. Aguilar-Cuenca R, et al. Cell Mol Life Sci. 2014 Feb;71(3):479-92. doi: 10.1007/s00018-013-1439-5. Epub 2013 Aug 11. Cell Mol Life Sci. 2014. PMID: 23934154 Free PMC article. Review. - Myosin regulatory light chains are required to maintain the stability of myosin II and cellular integrity.
Park I, Han C, Jin S, Lee B, Choi H, Kwon JT, Kim D, Kim J, Lifirsu E, Park WJ, Park ZY, Kim DH, Cho C. Park I, et al. Biochem J. 2011 Feb 15;434(1):171-80. doi: 10.1042/BJ20101473. Biochem J. 2011. PMID: 21126233 - Supervillin slows cell spreading by facilitating myosin II activation at the cell periphery.
Takizawa N, Ikebe R, Ikebe M, Luna EJ. Takizawa N, et al. J Cell Sci. 2007 Nov 1;120(Pt 21):3792-803. doi: 10.1242/jcs.008219. Epub 2007 Oct 9. J Cell Sci. 2007. PMID: 17925381 - Myosin phosphatase target subunit: Many roles in cell function.
Matsumura F, Hartshorne DJ. Matsumura F, et al. Biochem Biophys Res Commun. 2008 Apr 25;369(1):149-56. doi: 10.1016/j.bbrc.2007.12.090. Epub 2007 Dec 26. Biochem Biophys Res Commun. 2008. PMID: 18155661 Free PMC article. Review.
Cited by
- Suppression of non-muscle myosin II boosts T cell cytotoxicity against tumors.
Yang Y, Wen D, Lin F, Song X, Pang R, Sun W, Yu D, Zhang Z, Yu T, Kong J, Zhang L, Cao X, Liao W, Wang D, Yang Q, Liang J, Zhang N, Li K, Xiong C, Liu Y. Yang Y, et al. Sci Adv. 2024 Nov;10(44):eadp0631. doi: 10.1126/sciadv.adp0631. Epub 2024 Nov 1. Sci Adv. 2024. PMID: 39485850 Free PMC article. - Preliminary proteomic analysis of mouse lung tissue treated with cyclophosphamide and Venetin-1.
Czaplewska P, Müller M, Musiał N, Okrój M, Felberg-Miętka A, Sadowska J, Dudzińska W, Lubkowska A, Tokarz-Deptuła B, Fiołka M. Czaplewska P, et al. Sci Rep. 2024 Oct 23;14(1):25056. doi: 10.1038/s41598-024-76143-0. Sci Rep. 2024. PMID: 39443613 Free PMC article. - Emerging roles of cytoskeletal transport and scaffold systems in human viral propagation.
Lim Y, Cho YB, Seo YJ. Lim Y, et al. Anim Cells Syst (Seoul). 2024 Oct 21;28(1):506-518. doi: 10.1080/19768354.2024.2418332. eCollection 2024. Anim Cells Syst (Seoul). 2024. PMID: 39439927 Free PMC article. Review. - Editorial: Editors' showcase 2023: insights in cell adhesion and migration.
Horowitz A, Mammoto A, Sytnyk V, Jakovcevski I. Horowitz A, et al. Front Cell Dev Biol. 2024 Oct 3;12:1497689. doi: 10.3389/fcell.2024.1497689. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39421022 Free PMC article. No abstract available.
References
- Holmes KC. In: Myosins. Coluccio LM, editor. The Netherlands: Springer; 2007. pp. 35–54.Provides an in depth survey of selective myosin class members, including NM IIs. See also references , , , and .
- Mooseker MS, Foth BJ. In: Myosins. Coluccio LM, editor. The Netherlands: Springer; 2007. pp. 1–34.
- El-Mezgueldi M, Bagshaw CR. In: Myosins. Coluccio LM, editor. The Netherlands: Springer; 2007. pp. 55–93.
- Richards TA, Cavalier-Smith T. Myosin domain evolution and the primary divergence of eukaryotes. Nature. 2005;436:1113–1118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM023244-34/GM/NIGMS NIH HHS/United States
- U54 GM064346/GM/NIGMS NIH HHS/United States
- GM23244/GM/NIGMS NIH HHS/United States
- U54 GM064346-09/GM/NIGMS NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R37 GM023244/GM/NIGMS NIH HHS/United States
- U54-GM064346/GM/NIGMS NIH HHS/United States
- R01 GM023244/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases