Telomere shortening relaxes X chromosome inactivation and forces global transcriptome alterations - PubMed (original) (raw)
Telomere shortening relaxes X chromosome inactivation and forces global transcriptome alterations
Stefan Schoeftner et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Telomeres are heterochromatic structures at chromosome ends essential for chromosomal stability. Telomere shortening and the accumulation of dysfunctional telomeres are associated with organismal aging. Using telomerase-deficient TRF2-overexpressing mice (K5TRF2/Terc(-/-)) as a model for accelerated aging, we show that telomere shortening is paralleled by a gradual deregulation of the mammalian transcriptome leading to cumulative changes in a defined set of genes, including up-regulation of the mTOR and Akt survival pathways and down-regulation of cell cycle and DNA repair pathways. Increased DNA damage from dysfunctional telomeres leads to reduced deposition of H3K27me3 onto the inactive X chromosome (Xi), impaired association of the Xi with telomeric transcript accumulations (Tacs), and reactivation of an X chromosome-linked K5TRF2 transgene that is subjected to X-chromosome inactivation in female mice with sufficiently long telomeres. Exogenously induced DNA damage also disrupts Xi-Tacs, suggesting DNA damage at the origin of these alterations. Collectively, these findings suggest that critically short telomeres activate a persistent DNA damage response that alters gene expression programs in a nonstochastic manner toward cell cycle arrest and activation of survival pathways, as well as impacts the maintenance of epigenetic memory and nuclear organization, thereby contributing to organismal aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
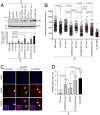
An X-linked transgene is re-expressed upon telomere shortening. (A) Male PM K5TRF2 mice display elevated TRF2 protein levels compared with female littermates. Actin, loading control. (B) Quantification of Western blots; n, number of keratinocyte preparations; standard error is indicated. A Student's t test was used to calculate statistical significance. (C) Skin phenotypes in PM K5TRF2/_Terc_−/− females. (D) Quantification of skin disorders. (E) Quantification of abnormalities in stratified epithelia. Female mice: wild type, n = 68; K5TRF2, n = 74; _G1 Terc_−/−, n = 34; K5TRF2/_G1 Terc_−/−, n = 32; _G2 Terc_−/−, n = 23; K5TRF2/_G2 Terc_−/−, n = 44; _G3 Terc_−/−, n = 4; K5TRF2/_G3 Terc_−/−, n = 13. Male mice: wild type, n = 24; K5TRF2, n = 7. A Fisher's exact test was used to calculate statistical significance. (F) Histopathological findings in stratified epithelia. Black arrowheads, displastic nuclei; white double-headed arrows, hyperplastic areas.
Fig. 2.
Loss of silencing of the X-linked PM K5TRF2 transgene leads to telomere dysfunction in K5TRF2/_Terc_−/− females. (A) (Top) TRF2 protein levels in back skin keratinocytes; (Bottom) quantification of TRF2 levels after normalizing against β-actin. n, 2–4 experiments. (B) Telomere Q-FISH in tail skin. Red bars, average telomere fluorescence intensity. A Student's t test was used to calculate statistical significance. a.f.u, arbitrary fluorescence units. (C) γH2AX-positive cells (white arrowheads) in the indicated skin sections. (Scale bar: 10 μm.) (D) Quantification of γH2AX immunostainings. n, mice analyzed; γH2AX-positive nuclei and total number of cells analyzed are indicated. A Fisher's exact test was used to calculate statistical significance.
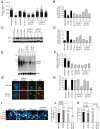
Fig. 3.
Critical telomere shortening results in impaired H3K27me3 of the Xi in vivo. (A) Combined immunostaining for H3K27me3 and RNA polymerase II in E12 embryo skin sections. (B) Reduced deposition of H3K27me3 on the Xi in K5TRF2/_G2 Terc_−/− skin sections. (C) Quantification of RNA polymerase II exclusion at the Xi (decorated with H3K27me3). (D) Immunostaining for H3K27me3 in adult tail-skin sections. (E) Reduced frequency of Xi-coupled H3K27me3 in adult skin sections of K5TRF2/_G2 Terc_−/− females. N, number of mice; n, nuclei analyzed. Error bars, standard error. An unpaired Student's t test was used to calculate statistical significance.
Fig. 4.
Critical telomere shortening results in a loss of _Xist_-Tacs association. (A) Distributions of individual telomere lengths in metaphases from primary keratinocytes. a.f.u., arbitrary fluorescence units; red bars, medium telomere fluorescence. (B) Average telomere length per genotype. Average values are shown on top of the bars. (C) TRF2 protein expression. (D) Quantification of TRF2 levels normalized against β-actin. (E) TelRNA/TERRA levels as detected by Northern blotting. (F) Quantification of TelRNA/TERRA levels normalized against gapdh. n, cell lines analyzed; standard error is indicated. (G) Combined _Xist_-TelRNA/TERRA RNA FISH to detect _Xist_-Tac association. Cells shown are polyploid and present 2 Xist signals. DNA was stained with DAPI. (H) _Xist_-TelRNA association is lost in most cultures with short telomeres and high TRF2. n, cell lines analyzed; standard error is indicated. (I) _Xist_-TelRNA RNA FISH in E12 embryonic skin sections. Green, Xist RNA; red, TelRNA/TERRA; blue, DAPI. Gray arrowheads, telomere-associated TelRNA/TERRA; white arrowheads, TelRNA accumulations (Tacs) in the vicinity of the _Xist_-coated inactive X chromosome. (J) K5TRF2/_G2 Terc_−/− E13 embryos display reduced Xi-Tac association. (K) Quantification of Tac frequency in E12 embryos. N, number of mice; n, nuclei analyzed. Error bars, standard error. An unpaired Student's t test was used to calculate statistical significance.
Fig. 5.
Deregulation of the mouse transcriptome is linked to progressive telomere shortening. (A) Venn diagrams showing overlapping gene expression patterns between the indicated pairwise comparisons of transcriptomes. Telomere shortening affects the expression of a similar group of genes between different pairwise comparisons. Red circles, down-regulated genes; green circles, up-regulated genes. (B) Transcript levels ratios of 20 probe sets robustly up-regulated or down-regulated in K5TRF2/_G3 Terc_−/− versus wild-type comparisons. Probe sets are ranked according to the false detection rate (FDR <1.00E-07; also see
Tables S2
and
S3
). Changes in transcript levels are tendentially reduced when the difference in telomere length between experimental samples is reduced. K5TRF2/_G2 Terc_−/− show only limited transcriptome changes when compared with wild-type keratinocytes.
Similar articles
- Telomerase abrogation dramatically accelerates TRF2-induced epithelial carcinogenesis.
Blanco R, Muñoz P, Flores JM, Klatt P, Blasco MA. Blanco R, et al. Genes Dev. 2007 Jan 15;21(2):206-20. doi: 10.1101/gad.406207. Genes Dev. 2007. PMID: 17234886 Free PMC article. - Role of TRF2 in the assembly of telomeric chromatin.
Benetti R, Schoeftner S, Muñoz P, Blasco MA. Benetti R, et al. Cell Cycle. 2008 Nov 1;7(21):3461-8. doi: 10.4161/cc.7.21.7013. Epub 2008 Nov 12. Cell Cycle. 2008. PMID: 18971622 - DNA-PKcs-interacting protein KIP binding to TRF2 is required for the maintenance of functional telomeres.
Khadka P, Lee JH, Baek SH, Oh SY, Chung IK. Khadka P, et al. Biochem J. 2014 Oct 1;463(1):19-30. doi: 10.1042/BJ20131395. Biochem J. 2014. PMID: 25012820 - Mammalian telomeres and telomerase: why they matter for cancer and aging.
Blasco MA. Blasco MA. Eur J Cell Biol. 2003 Sep;82(9):441-6. doi: 10.1078/0171-9335-00335. Eur J Cell Biol. 2003. PMID: 14582532 Review. - The Connection Between Cell Fate and Telomere.
Engin AB, Engin A. Engin AB, et al. Adv Exp Med Biol. 2021;1275:71-100. doi: 10.1007/978-3-030-49844-3_3. Adv Exp Med Biol. 2021. PMID: 33539012 Review.
Cited by
- Sex as a biological variable in ageing: insights and perspectives on the molecular and cellular hallmarks.
Fritz García JHG, Keller Valsecchi CI, Basilicata MF. Fritz García JHG, et al. Open Biol. 2024 Oct;14(10):240177. doi: 10.1098/rsob.240177. Epub 2024 Oct 30. Open Biol. 2024. PMID: 39471841 Free PMC article. Review. - RBMX involves in telomere stability maintenance by regulating TERRA expression.
Liu J, Zheng T, Chen D, Huang J, Zhao Y, Ma W, Liu H. Liu J, et al. PLoS Genet. 2023 Sep 27;19(9):e1010937. doi: 10.1371/journal.pgen.1010937. eCollection 2023 Sep. PLoS Genet. 2023. PMID: 37756323 Free PMC article. - Short telomeres impede germ cell specification by upregulating MAPK and TGFβ signaling.
Tian C, Heng D, Zhao N, Liu L, Sheng X, Chen J, Liu L. Tian C, et al. Sci China Life Sci. 2023 Feb;66(2):324-339. doi: 10.1007/s11427-022-2151-0. Epub 2022 Sep 16. Sci China Life Sci. 2023. PMID: 36125668 - The X in seX-biased immunity and autoimmune rheumatic disease.
Jiwrajka N, Anguera MC. Jiwrajka N, et al. J Exp Med. 2022 Jun 6;219(6):e20211487. doi: 10.1084/jem.20211487. Epub 2022 May 5. J Exp Med. 2022. PMID: 35510951 Free PMC article. Review. - HSF1-Activated Non-Coding Stress Response: Satellite lncRNAs and Beyond, an Emerging Story with a Complex Scenario.
Vourc'h C, Dufour S, Timcheva K, Seigneurin-Berny D, Verdel A. Vourc'h C, et al. Genes (Basel). 2022 Mar 27;13(4):597. doi: 10.3390/genes13040597. Genes (Basel). 2022. PMID: 35456403 Free PMC article. Review.
References
- Chan SW, Blackburn EH. New ways not to make ends meet: Telomerase, DNA damage proteins and heterochromatin. Oncogene. 2002;21:553–563. - PubMed
- de Lange T. Protection of mammalian telomeres. Oncogene. 2002;21:532–540. - PubMed
- Blasco MA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. - PubMed
- d'Adda di Fagagna F, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. - PubMed
- de Lange T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous