Administration of S-nitrosoglutathione after traumatic brain injury protects the neurovascular unit and reduces secondary injury in a rat model of controlled cortical impact - PubMed (original) (raw)
Administration of S-nitrosoglutathione after traumatic brain injury protects the neurovascular unit and reduces secondary injury in a rat model of controlled cortical impact
Mushfiquddin Khan et al. J Neuroinflammation. 2009.
Abstract
Background: Traumatic brain injury (TBI) is a major cause of preventable death and serious morbidity in young adults. This complex pathological condition is characterized by significant blood brain barrier (BBB) leakage that stems from cerebral ischemia, inflammation, and redox imbalances in the traumatic penumbra of the injured brain. Once trauma has occurred, combating these exacerbations is the keystone of an effective TBI therapy. Following other brain injuries, nitric oxide modulators such as S-nitrosoglutathione (GSNO) maintain not only redox balance but also inhibit the mechanisms of secondary injury. Therefore, we tested whether GSNO shows efficacy in a rat model of experimental TBI.
Methods: TBI was induced by controlled cortical impact (CCI) in adult male rats. GSNO (50 microg/kg body weight) was administered at two hours after CCI. GSNO-treated injured animals (CCI+GSNO group) were compared with vehicle-treated injured animals (CCI+VEH group) in terms of tissue morphology, BBB leakage, edema, inflammation, cell death, and neurological deficit.
Results: Treatment of the TBI animals with GSNO reduced BBB disruption as evidenced by decreased Evan's blue extravasation across brain, infiltration/activation of macrophages (ED1 positive cells), and reduced expression of ICAM-1 and MMP-9. The GSNO treatment also restored CCI-mediated reduced expression of BBB integrity proteins ZO-1 and occludin. GSNO-mediated improvements in tissue histology shown by reduction of lesion size and decreased loss of both myelin (measured by LFB staining) and neurons (assayed by TUNEL) further support the efficacy of GSNO therapy. GSNO-mediated reduced expression of iNOS in macrophages as well as decreased neuronal cell death may be responsible for the histological improvement and reduced exacerbations. In addition to these biochemical and histological improvements, GSNO-treated injured animals recovered neurobehavioral functions as evaluated by the rotarod task and neurological score measurements.
Conclusion: GSNO is a promising candidate to be evaluated in humans after brain trauma because it not only protects the traumatic penumbra from secondary injury and improves overall tissue structure but also maintains the integrity of BBB and reduces neurologic deficits following CCI in a rat model of experimental TBI.
Figures
Figure 1
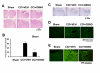
Effect of GSNO treatment on TBI-mediated tissue injury and on the expression of ICAM-1 and ED1 in ipsilateral rat brain after CCI. Animals were treated with GSNO (0.05 mg/kg) 2 h following CCI. Seven days after TBI, brains were removed, sectioned and stained with H&E (A) and LFB (C) and analyzed for lesion size (B) using morphometric measurement. GSNO reduced TBI-mediated tissue injury, decreased lesion size (B), and reduced the expression of ICAM-1 (D) and ED1 (E) (detected in traumatic penumbral area at 72 h following TBI). Photomicrographs are the representative of n = 3 in each group. Lesion size data are presented as mean ± SD (n = 3). ***p < 0.001 vs. CCI+VEH.
Figure 2
Effect of GSNO on TBI-mediated BBB disruption, edema and on the expression of ZO-1, occludin and MMP-9 in ipsilateral rat brain after CCI. Representative photographs showing Evan's blue extravasation at 48 h in 2 out of 6 coronal sections (A). Significant EB leakage was observed in CCI+VEH brain, and the leakage was reduced in the CCI+GSNO group. EB extravasation was not observed in Sham animals (see Table 1). A similar treatment with GSNO decreased the brain water content (edema) in ipsilateral brain (C) measured at 24 h after CCI. Treatment with GSNO also enhanced TBI-mediated reduced expression of ZO-1 and occludin in traumatic penumbral area from ipsilateral hemisphere measures at 24 h following TBI by Western blot (B). TBI-mediated increased expression of MMP-9 was also decreased following GSNO treatment (detected in traumatic penumbral area at 72 h following TBI) (D). Photomicrograph of Evan's blue is the representative of n = 5. Edema data are presented as mean ± SD (n = 5). Western blot and photomicrographs of MMP-9 immunohistochemistry are representative of n = 3 in each group. *p < 0.05 vs. CCI+VEH or Sham. ips, ipsilateral; conr, contralateral.
Figure 3
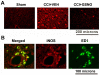
Effect of GSNO on the expression of iNOS present mainly in macrophages/microglia. Red fluorescence indicates induced higher expression of iNOS in the traumatic penumbral area of CCI+VEH brain than in the treated (CCI+GSNO) brain determined by immunohistochemistry at 72 h following CCI. Sham animal did not show any significant staining of iNOS (A). Colocalization of the expression of iNOS with ED1 as yellowish fluorescence (B) indicates that iNOS was mainly expressed in macrophages/microglia.
Figure 4
Effect of GSNO on TBI-mediated deficits in neurobehavioral function. A treatment at 2 h post-injury following CCI with GSNO improved motor function evaluated by rotarod task (A), decreased modified neurological severity score (mNSS) (B) evaluated on 6th day after CCI and reduced time to remove the stimuli from limbs (tactile strength test) (C). Data are presented as mean ± SD (n = 7). ***p < 0.001, **p < 0.01, *p < 0.05 vs. CCI+VEH.
Figure 5
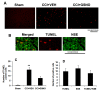
Effect of GSNO on neuronal apoptotic cell death (TUNEL assay) at 72 h after CCI. Traumatic penumbral region in CCI+VEH brain had a significantly higher number of TUNEL positive cells compared to CCI+GSNO treated brain. Sham brain had no TUNEL positive cells (A). TUNEL positive cells were found to colocalize with neuronal marker NSE (B). Number of TUNEL positive cells (C) and number of stained positive cells (D) were counted in three different fields and averaged. Data are presented as mean ± SD TUNEL staining was carried out as described in Methods. Photomicrographs are the representative of n = 3 in each group. **p < 0.01 vs. Sham and CCI+GSNO.
Similar articles
- S-nitrosoglutathione reduces oxidative injury and promotes mechanisms of neurorepair following traumatic brain injury in rats.
Khan M, Sakakima H, Dhammu TS, Shunmugavel A, Im YB, Gilg AG, Singh AK, Singh I. Khan M, et al. J Neuroinflammation. 2011 Jul 6;8:78. doi: 10.1186/1742-2094-8-78. J Neuroinflammation. 2011. PMID: 21733162 Free PMC article. - Administration of palmitoylethanolamide (PEA) protects the neurovascular unit and reduces secondary injury after traumatic brain injury in mice.
Ahmad A, Crupi R, Impellizzeri D, Campolo M, Marino A, Esposito E, Cuzzocrea S. Ahmad A, et al. Brain Behav Immun. 2012 Nov;26(8):1310-21. doi: 10.1016/j.bbi.2012.07.021. Epub 2012 Aug 3. Brain Behav Immun. 2012. PMID: 22884901 - The inhibitory effect of S-nitrosoglutathione on blood-brain barrier disruption and peroxynitrite formation in a rat model of experimental stroke.
Khan M, Dhammu TS, Sakakima H, Shunmugavel A, Gilg AG, Singh AK, Singh I. Khan M, et al. J Neurochem. 2012 Nov;123 Suppl 2(Suppl 2):86-97. doi: 10.1111/j.1471-4159.2012.07947.x. J Neurochem. 2012. PMID: 23050646 Free PMC article. - Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury.
Shlosberg D, Benifla M, Kaufer D, Friedman A. Shlosberg D, et al. Nat Rev Neurol. 2010 Jul;6(7):393-403. doi: 10.1038/nrneurol.2010.74. Epub 2010 Jun 15. Nat Rev Neurol. 2010. PMID: 20551947 Free PMC article. Review. - Matrix Metalloproteinase-9 inhibitors as therapeutic drugs for traumatic brain injury.
Sunny A, James RR, Menon SR, Rayaroth S, Daniel A, Thompson NA, Tharakan B. Sunny A, et al. Neurochem Int. 2024 Jan;172:105642. doi: 10.1016/j.neuint.2023.105642. Epub 2023 Nov 24. Neurochem Int. 2024. PMID: 38008261 Review.
Cited by
- Apelin-13 as a novel target for intervention in secondary injury after traumatic brain injury.
Bao HJ, Qiu HY, Kuai JX, Song CJ, Wang SX, Wang CQ, Peng HB, Han WC, Wu YP. Bao HJ, et al. Neural Regen Res. 2016 Jul;11(7):1128-33. doi: 10.4103/1673-5374.187049. Neural Regen Res. 2016. PMID: 27630697 Free PMC article. - Neutrophil depletion reduces edema formation and tissue loss following traumatic brain injury in mice.
Kenne E, Erlandsson A, Lindbom L, Hillered L, Clausen F. Kenne E, et al. J Neuroinflammation. 2012 Jan 23;9:17. doi: 10.1186/1742-2094-9-17. J Neuroinflammation. 2012. PMID: 22269349 Free PMC article. - GSNO promotes functional recovery in experimental TBI by stabilizing HIF-1α.
Khan M, Dhammu TS, Baarine M, Kim J, Paintlia MK, Singh I, Singh AK. Khan M, et al. Behav Brain Res. 2018 Mar 15;340:63-70. doi: 10.1016/j.bbr.2016.10.037. Epub 2016 Oct 22. Behav Brain Res. 2018. PMID: 27780722 Free PMC article. - Protective role of S-nitrosoglutathione (GSNO) against cognitive impairment in rat model of chronic cerebral hypoperfusion.
Won JS, Kim J, Annamalai B, Shunmugavel A, Singh I, Singh AK. Won JS, et al. J Alzheimers Dis. 2013;34(3):621-35. doi: 10.3233/JAD-121786. J Alzheimers Dis. 2013. PMID: 23254638 Free PMC article. - Lycium barbarum extracts protect the brain from blood-brain barrier disruption and cerebral edema in experimental stroke.
Yang D, Li SY, Yeung CM, Chang RC, So KF, Wong D, Lo AC. Yang D, et al. PLoS One. 2012;7(3):e33596. doi: 10.1371/journal.pone.0033596. Epub 2012 Mar 16. PLoS One. 2012. PMID: 22438957 Free PMC article.
References
- Coles JP, Fryer TD, Smielewski P, Chatfield DA, Steiner LA, Johnston AJ, Downey SP, Williams GB, Aigbirhio F, Hutchinson PJ, et al. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J Cereb Blood Flow Metab. 2004;24:202–211. doi: 10.1097/01.WCB.0000103022.98348.24. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS022576/NS/NINDS NIH HHS/United States
- R01 NS034741/NS/NINDS NIH HHS/United States
- R37 NS022576/NS/NINDS NIH HHS/United States
- NS-22576/NS/NINDS NIH HHS/United States
- C06 RR018823/RR/NCRR NIH HHS/United States
- C06 RR015455/RR/NCRR NIH HHS/United States
- NS-37766/NS/NINDS NIH HHS/United States
- R01 NS037766/NS/NINDS NIH HHS/United States
- NS-34741/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous