Does neuroinflammation fan the flame in neurodegenerative diseases? - PubMed (original) (raw)
Does neuroinflammation fan the flame in neurodegenerative diseases?
Tamy C Frank-Cannon et al. Mol Neurodegener. 2009.
Abstract
While peripheral immune access to the central nervous system (CNS) is restricted and tightly controlled, the CNS is capable of dynamic immune and inflammatory responses to a variety of insults. Infections, trauma, stroke, toxins and other stimuli are capable of producing an immediate and short lived activation of the innate immune system within the CNS. This acute neuroinflammatory response includes activation of the resident immune cells (microglia) resulting in a phagocytic phenotype and the release of inflammatory mediators such as cytokines and chemokines. While an acute insult may trigger oxidative and nitrosative stress, it is typically short-lived and unlikely to be detrimental to long-term neuronal survival. In contrast, chronic neuroinflammation is a long-standing and often self-perpetuating neuroinflammatory response that persists long after an initial injury or insult. Chronic neuroinflammation includes not only long-standing activation of microglia and subsequent sustained release of inflammatory mediators, but also the resulting increased oxidative and nitrosative stress. The sustained release of inflammatory mediators works to perpetuate the inflammatory cycle, activating additional microglia, promoting their proliferation, and resulting in further release of inflammatory factors. Neurodegenerative CNS disorders, including multiple sclerosis (MS), Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), amyotrophic lateral sclerosis (ALS), tauopathies, and age-related macular degeneration (ARMD), are associated with chronic neuroinflammation and elevated levels of several cytokines. Here we review the hallmarks of acute and chronic inflammatory responses in the CNS, the reasons why microglial activation represents a convergence point for diverse stimuli that may promote or compromise neuronal survival, and the epidemiologic, pharmacologic and genetic evidence implicating neuroinflammation in the pathophysiology of several neurodegenerative diseases.
Figures
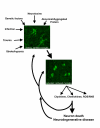
Figure 1
Neuroinflammation can precede and contribute to neuronal dysfunction and degeneration. Divergent initiating triggers directly or indirectly converge to activate microglia (stained here with an antibody against F4/80) from a ramified/resting state to an ameboid-shape/activated state, initiating a self-propelling cycle of neuroinflammation and chronic over-production of inflammatory mediators. These mediators impact susceptible neuronal populations in the CNS and contribute to their demise within the context of each neurodegenerative disorder. The progressive loss of neurons that characterizes these disorders further contributes to generation of debris and keeps microglia activated indefinitely maintaining microglia in an activated state long-term.
Similar articles
- Metabolic Control of Glia-Mediated Neuroinflammation.
Jha MK, Park DH, Kook H, Lee IK, Lee WH, Suk K. Jha MK, et al. Curr Alzheimer Res. 2016;13(4):387-402. doi: 10.2174/1567205013666151116124755. Curr Alzheimer Res. 2016. PMID: 26567748 Review. - Inflammatory Mechanisms and Oxidative Stress as Key Factors Responsible for Progression of Neurodegeneration: Role of Brain Innate Immune System.
Leszek J, Barreto GE, Gąsiorowski K, Koutsouraki E, Ávila-Rodrigues M, Aliev G. Leszek J, et al. CNS Neurol Disord Drug Targets. 2016;15(3):329-36. doi: 10.2174/1871527315666160202125914. CNS Neurol Disord Drug Targets. 2016. PMID: 26831258 Review. - Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer's disease.
Singh D. Singh D. J Neuroinflammation. 2022 Aug 17;19(1):206. doi: 10.1186/s12974-022-02565-0. J Neuroinflammation. 2022. PMID: 35978311 Free PMC article. Review. - The effects of statins on microglial cells to protect against neurodegenerative disorders: A mechanistic review.
Bagheri H, Ghasemi F, Barreto GE, Sathyapalan T, Jamialahmadi T, Sahebkar A. Bagheri H, et al. Biofactors. 2020 May;46(3):309-325. doi: 10.1002/biof.1597. Epub 2019 Dec 17. Biofactors. 2020. PMID: 31846136 Review. - Effects of Curcumin on Microglial Cells.
Ghasemi F, Bagheri H, Barreto GE, Read MI, Sahebkar A. Ghasemi F, et al. Neurotox Res. 2019 Jul;36(1):12-26. doi: 10.1007/s12640-019-00030-0. Epub 2019 Apr 4. Neurotox Res. 2019. PMID: 30949950 Review.
Cited by
- Degeneration of Dopaminergic Neurons Due to Metabolic Alterations and Parkinson's Disease.
Song J, Kim J. Song J, et al. Front Aging Neurosci. 2016 Mar 30;8:65. doi: 10.3389/fnagi.2016.00065. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27065205 Free PMC article. Review. - The Anti-Neuroinflammatory Effect of Fuzi and Ganjiang Extraction on LPS-Induced BV2 Microglia and Its Intervention Function on Depression-Like Behavior of Cancer-Related Fatigue Model Mice.
Yang S, Yang Y, Chen C, Wang H, Ai Q, Lin M, Zeng Q, Zhang Y, Gao Y, Li X, Chen N. Yang S, et al. Front Pharmacol. 2021 May 28;12:670586. doi: 10.3389/fphar.2021.670586. eCollection 2021. Front Pharmacol. 2021. PMID: 34122094 Free PMC article. - Role of neuroinflammation in adult neurogenesis and Alzheimer disease: therapeutic approaches.
Fuster-Matanzo A, Llorens-Martín M, Hernández F, Avila J. Fuster-Matanzo A, et al. Mediators Inflamm. 2013;2013:260925. doi: 10.1155/2013/260925. Epub 2013 Apr 3. Mediators Inflamm. 2013. PMID: 23690659 Free PMC article. Review. - Command Suicidal Hallucination as Initial Presentation of Coronavirus Disease 2019 (COVID-19): A Case Report.
Mirza J, Ganguly A, Ostrovskaya A, Tusher A, Viswanathan R. Mirza J, et al. Psychosomatics. 2020 Sep-Oct;61(5):561-564. doi: 10.1016/j.psym.2020.05.022. Epub 2020 May 30. Psychosomatics. 2020. PMID: 32593478 Free PMC article. No abstract available. - Microglia in Prion Diseases: Angels or Demons?
Peggion C, Stella R, Lorenzon P, Spisni E, Bertoli A, Massimino ML. Peggion C, et al. Int J Mol Sci. 2020 Oct 20;21(20):7765. doi: 10.3390/ijms21207765. Int J Mol Sci. 2020. PMID: 33092220 Free PMC article. Review.
References
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9(6):429–39. - PubMed
- Crutcher KA, Gendelman HE, Kipnis J, Perez-Polo JR, Perry VH, Popovich PG, Weaver LC. Debate: "is increasing neuroinflammation beneficial for neural repair?". J Neuroimmune Pharmacol. 2006;1(3):195–211. - PubMed
- Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9(6):481–93. - PubMed
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76(2):77–98. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous