Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis - PubMed (original) (raw)
Comparative Study
Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis
Sacri R Ferrón et al. J Neurosci. 2009.
Abstract
Proliferation in the subependymal zone (SEZ) and neurogenesis in the olfactory bulb decline in the forebrain of telomerase-deficient mice. The present work reveals additional effects of telomere shortening on neuronal differentiation, as adult multipotent progenitors with critically short telomeres yield reduced numbers of neurons that, furthermore, exhibit underdeveloped neuritic arbors. Genetic data indicate that the tumor suppressor protein p53 not only mediates the adverse effects of telomere attrition on proliferation and self-renewal but it is also involved in preventing normal neuronal differentiation of adult progenitors with dysfunctional telomeres. Interestingly, progenitor cells with short telomeres obtained from fetal brains do not exhibit any replicative defects but also fail to acquire a fully mature neuritic arbor, demonstrating cell cycle-independent effects of telomeres on neuronal differentiation. The negative effect of p53 on neuritogenesis is mechanistically linked to its cooperation with the Notch pathway in the upregulation of small GTPase RhoA kinases, Rock1 and Rock2, suggesting a potential link between DNA damage and the Notch signaling pathway in the control of neuritogenesis. We also show that telomerase expression is downregulated in the SEZ of aging mice leading to telomere length reductions in neurosphere-forming cells and deficient neurogenesis and neuritogenesis. Our results suggest that age-related deficits could be caused partly by dysfunctional telomeres and demonstrate that p53 is a central modulator of adult neurogenesis, regulating both the production and differentiation of postnatally generated olfactory neurons.
Figures
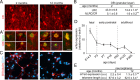
Figure 1.
Telomere shortening and neuritogenesis defects are associated with aging. A, Fluorescent micrographs showing the labeling of granular neurons with anti-CR antibodies in the OB of 2- and 12-month-old mice. A′, High-power micrographs showing newly born CR+ neurons (red), which have incorporated and retained BrdU (green) injected 1 month before the killing of the animal. Notice that CR+ neurons appear less differentiated in old animals. B, Table showing the relative number of BrdU-LRCs and the percentage of CR+ BrdU-LRCs in the granular layer of the OB in 2- and 12-month-old mice (n = 3 mice per age). C, βIII-tubulin+ neurons differentiating in neurosphere cultures obtained from 12-month-old mice exhibit shorter and less developed neuritic arbors when compared with cultures from 2-month-old mice. D, Measurement of the levels of the mRNA of the enzyme telomerase (Tert) relative to β-actin levels, in tissue homogenates from E14.5 ganglionic eminences and from the SEZ of early postnatal (P), adult, and aged mice (n = 3 mice per age). E, Table showing the quantification of the levels of the mRNA of Tert relative to β-actin levels and the relative length of telomeres in neurosphere cells isolated from 2- and 12-month-old mice (n = 3 independent cultures per age; a.u., arbitrary units). Error bars indicate SEM. *p < 0.05, **p < 0.01. Scale bars: A, 30 μm; A′, 10 μm; C, 30 μm.
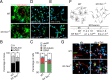
Figure 2.
Removal of p53 is sufficient to rescue defects in proliferation, self-renewal, and differentiation in the SEZ of telomerase-deficient mice. A, Table showing the number of BrdU-LRCs and the percentage of CR+ LRCs in the granular and periglomerular layers of the OB of WT, G3 _Terc_−/−, G3 Terc_−/−;p53_−/−, and p53 −/− 2-month-old mice. B, Immunofluorescent detection of CR+ interneurons (red) in the granular layer of the OB of WT, G3 _Terc_−/−, G3 Terc_−/−;p53_−/−, and _p53_−/− 2-month-old mice. Notice that the reduction in the density of CR+ neurons in single Terc mutants is rescued by the p53 loss. DAPI (blue) is used as a nuclear staining. C, Graph showing the average length of the longest dendrite per CR+ neurons in the granular layer of the OB in mice of different genotypes (n = 3 mice per genotype). D, Fluorescent micrographs from WT, G3 _Terc_−/−, G3 Terc_−/−;p53_−/−, and p53 −/− SEZs immunostained with anti-GFAP (blue) and anti-BrdU (red) antibodies and counterstained with Sytox (green). The limit with the lateral ventricle (V) is indicated by the white broken line. In white, the percentage of GFAP+ cells that have incorporated BrdU on the day of killing (7 injections; 1 every 2 h) ± SEM. E, Graph showing the total number of GFAP+/Sox2+/LRCs in the SEZ of WT, G3 _Terc_−/−, G3 Terc_−/−;p53_−/−, and p53 −/− 2-month-old mice (n = 3 mice per genotype). F, Phase contrast micrographs of primary neurospheres obtained from SEZ tissue of mice with different genotypes. The mean diameters (in micrometers ± SEM) are indicated in each photograph. G, Primary sphere formation in double p53 and telomerase-deficient animals (G3 Terc_−/−;p53_−/−) is normal relative to control animals (WT) (n = 3 independent cultures per genotype). *p < 0.05, **p < 0.01. Scale bars: C, 100 μm; D, 20 μm.
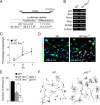
Figure 3.
Neurogenic and neuritogenic phenotype restoration in G3 Terc_−/−;p53_ cells. A, Table showing the percentages of GFAP+ and S100β+ astrocytes, βIII-tubulin+ neurons, and O4+ oligodendrocytes in differentiating cultures derived from WT, G3 _Terc_−/−, G3 Terc_−/−;p53_−/−, and _p53_−/− adult young mice. G3 _Terc_−/− cultures show a reduction in neurogenic potential that is rescued by the p53 deficiency (n = 3 cultures per genotype). B, Fluorescent micrographs of WT, G3 _Terc_−/−, G3 Terc_−/−;p53_−/−, and _p53_−/− cultures after 7 d of differentiation showing neurons (βIII-tubulin+; green) and astrocytes (GFAP+; red). C, Representative drawings of βIII-tubulin+ neurons differentiated from neurosphere cultures of different genotypes. Notice that mutant neurons are more stunted and less branched than wild types and that this defect is rescued by a p53 deficiency. D, Graphs showing quantifications of end points (EP) in βIII-tubulin+ neurons differentiated from WT, G3 _Terc_−/−, G3 Terc_−/−;p53_−/−, and _p53_−/− cells for 7 div (n = 3 cultures per genotype). Error bars indicate SEM. **p < 0.01. Scale bar: B, 30 μm.
Figure 4.
Reduced neurogenesis/neuritogenesis in eNSCs with short telomeres. A, Fluorescent micrographs of fetal cultures from WT and from G5 _Terc_−/− E14.5 embryos immunolabeled for the detection of neurons (βIII-tubulin+; red), astrocytes (GFAP+; green), and oligodendrocytes (O4; blue). DAPI is used as a nuclear staining. B, Proportions of wild-type and G5 _Terc_−/− neurospheres that produce three (tripotent), two (bipotent), or one (astrocyte-only) cell derivatives. There are no significant differences in the proportion of multipotent clones between wild-type and mutant cultures. C, Proportions of βIII-tubulin+, GFAP+, O4+ cells, and of undifferentiated (undiff.; negative for any of the three mentioned markers) cells in cultures differentiating from wild-type and from G5 _Terc_−/− cells (n = 3 cultures per genotype). Notice a significant reduction in the proportion of neurons in mutant cultures. D, Fluorescent micrographs of fetal cultures from WT and from G5 _Terc_−/− E14.5 embryos immunolabeled for the detection of MAP2 (red). DAPI is used as a nuclear staining. The percentage of positive cells relative to total numbers is indicated in white for each genotype. E, Micrographs showing the immunofluorescent detection of the marker for undifferentiated cells nestin in cultures differentiated for only 2 div. DAPI is used as a nuclear staining. The percentage of positive cells relative to total numbers is indicated in white for each genotype. F, Representative drawings of βIII-tubulin+ neurons differentiated from WT and G5 _Terc_−/− cells and table showing the numbers of neuritic end points (EP) in WT and G5 _Terc_−/− βIII-tubulin+ neurons after differentiating for 4 and 7 div (n = 3 cultures per genotype). Notice that mutant neurons are more stunted and have fewer branches than wild types. G, Fluorescent micrographs showing βIII-tubulin+ neurons (red) differentiated for 2 div from WT and G5 _Terc_−/− neurosphere cells that had been electroporated with gfp-mTert or with gfp alone (GFP+; green). Error bars indicate SEM. *p < 0.05, **p < 0.01. Scale bars: A, D, E, G, 20 μm.
Figure 5.
Reduced neuritogenesis in _Terc_−/− embryonic neurons correlates with p53 levels. A, Luciferase activity (in arbitrary units) in proliferating and differentiating WT and G5 _Terc_−/− fetal neurosphere cells transduced with the p53-responsive PIG3-luc reporter (n = 3 cultures/transfections per genotype). B, RT-PCR detection of transcript levels for several p53-downstream genes involved in the regulation of apoptosis in WT and G5 _Terc_−/− cells, showing unchanged levels in mutants. Cell cycle regulator p21, another p53 target, is increased, an additional indication that p53 activity is increased in mutant cells with short telomeres (n = 3 cultures per genotype). C, Graph showing quantification of cell apoptosis, as the percentage of caspase-3-positive cells in WT and G5 _Terc_−/− cells at 2, 4, or 7 div during differentiation (n = 3 cultures per genotype). Apoptotic cells are rare in these cultures, and their frequency is not significantly increased by a higher incidence in short telomeres. D, Differentiating fetal cultures immunostained for βIII-tubulin (green), p53 (red), and DAPI (blue). Neurons appear less differentiated in mutant cultures. Numbers in white indicate proportions of p53-immunopositive cells relative to total number of βIII-tubulin+ neurons in mutant and wild-type cultures (n = 3 cultures per genotype). E, Graph showing quantifications of end points (EP) in WT neurons and in G5 _Terc_−/− neurons that were either p53− or p53+ at the time of analysis, after 7 div of differentiation (n = 3 cultures per genotype). Notice that p53 immunoreactivity correlates with a more dramatic phenotype. F, Representative drawings of βIII-tubulin+ neurons isolated from the striata of WT and _p53_−/− E14.5 embryos and cultured for 7 div. Notice the increased number of EP/neuron in _p53_−/− striatal neurons. Error bars indicate SEM. *p < 0.05, **p < 0.01. Scale bar: D, 20 μm.
Figure 6.
p53 and Notch regulate neuritogenesis. A, Representative drawings of βIII-tubulin+ neurons differentiated from WT and _p53_−/− adult cells that were treated with the γ-secretase inhibitor at 1 μ
m
. B, Fluorescent micrographs from wild-type differentiated cultures showing the immunostaining with antibodies that specifically recognize the cleaved intracellular fragment of Notch (NICD) (green) and tubulin (red) and counterstained with DAPI (blue). Notice that γ-secretase inhibition abrogates the appearance of the nuclear NICD immunostaining. C, Graph showing quantifications of end points (EP) in WT and in _p53_−/− neurons differentiated for 7 div in the presence of 1 μ
m
L-685.458 (γ-secretase inhibitor). The untreated condition consisted in the addition of 0.1% DMSO in which the inhibitor was dissolved. EP values are expressed as fold increase relative to measurements in WT neurons in normal differentiation culture medium (indicated by the dashed line). Notice that the Notch pathway normally inhibits neuritogenesis in WT neurons but not in p53-mutant neurons. D, Graph showing quantifications of EP in WT and _p53_−/− neurons transduced with pMXIE or pMXIE-NotchIC constructs and differentiated for 7 div afterward, relative to wild-type values (WT) in the absence of any manipulation (dashed line). Constitutive activation of the Notch pathway does not further inhibit normal neuritogenesis, but it blocks arborization induced by the absence of p53. E, Graph showing quantifications of EPs in neurons in fetal WT and G5 _Terc_−/− cultures that had been treated with the γ-secretase inhibitor and differentiated for 7 div. The untreated condition consisted in the addition of 0.1% DMSO in which the inhibitor was dissolved. EP values are expressed as fold increase relative to measurements in WT neurons in normal differentiation culture medium (indicated by the dashed line). Notice that the treatment causes an increase in the arborization in WT and mutant neurons. F, Graph showing quantifications of EPs in neurons in adult WT and G3 _Terc_−/− cultures that had been treated with the γ-secretase inhibitor and differentiated for 7 div. EP values are expressed as fold increase relative to measurements in WT neurons in normal differentiation culture medium (indicated by the dashed line). Notice that the treatment causes an increase in the arborization in WT and mutant neurons. G, Semiquantitative PCR analysis of Notch1 and Notch3 genes in differentiating cultures from WT and p53−/− adult cells and from WT and G5 _Terc_−/− fetal cells. Notice that no differences were seen in any of the genotypes in the densitometric values relative to the intensity of the β-actin band used as a control. Error bars indicate SEM. *p < 0.05, **p < 0.01.
Figure 7.
p53 and Notch cooperate to regulate neuritogenesis through Rock1/2. A, Semiquantitative PCR analysis of Rock1 and Rock2 genes in WT and _p53_−/− adult differentiated cells transduced with pMXIE or pMXIE-NotchIC, as well as in WT and G5 _Terc_−/− fetal cultures. Statistical differences between control and NotchIC-transduced cells are indicated with an asterisk (*) and between genotypes with a pound sign (#). Lack of p53 results in reduced levels of both Rock1 and 2 and Notch overexpression upregulates the expression of both genes and compensates for the lack of p53. B, Representative drawings of βIII-tubulin+ neurons differentiated from WT and _p53_−/− adult cultures transduced with pMXIE or pMXIE-NotchIC, and treated or not with the Rock1/2 inhibitor H-1152 at 1 μ
m
. C, Graph showing quantification of EP in WT and in _p53_−/− neurons that were transduced with pMXIE or pMXIE-NotchIC and differentiated for 7 div with or without the Rock1/2 inhibitor. D, Graph showing quantification of neuronal end points (EP) in fetal WT and G5 _Terc_−/− cultures differentiated in the presence or in the absence of the Rock1/2 inhibitor. Notice that the reduction in the number of EP/neuron displayed by the mutant neurons is rescued when Rock1/2 are inhibited. E, Graph showing the quantification of neuronal end points (EP) in neurons in adult WT and G3 _Terc_−/− cultures differentiated in the presence or in the absence of the Rock1/2 inhibitor. Notice that the reduction in the number of EP/neuron displayed by the mutant neurons is rescued when Rock1/2 are inhibited. F, Proposed model to explain how telomere dysfunction-mediated p53 activation can interact with extracellular Notch to regulate neuritogenesis. Error bars indicate SEM. *p < 0.05, **p < 0.01; #p < 0.05.
Similar articles
- Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice.
Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadiñanos J, Horner JW, Maratos-Flier E, Depinho RA. Jaskelioff M, et al. Nature. 2011 Jan 6;469(7328):102-6. doi: 10.1038/nature09603. Epub 2010 Nov 28. Nature. 2011. PMID: 21113150 Free PMC article. - p53 regulates the self-renewal and differentiation of neural precursors.
Armesilla-Diaz A, Bragado P, Del Valle I, Cuevas E, Lazaro I, Martin C, Cigudosa JC, Silva A. Armesilla-Diaz A, et al. Neuroscience. 2009 Feb 18;158(4):1378-89. doi: 10.1016/j.neuroscience.2008.10.052. Epub 2008 Nov 7. Neuroscience. 2009. PMID: 19038313 - Pulmonary Alveolar Stem Cell Senescence, Apoptosis, and Differentiation by p53-Dependent and -Independent Mechanisms in Telomerase-Deficient Mice.
Zhang K, Wang L, Hong X, Chen H, Shi Y, Liu Y, Liu J, Liu JP. Zhang K, et al. Cells. 2021 Oct 26;10(11):2892. doi: 10.3390/cells10112892. Cells. 2021. PMID: 34831112 Free PMC article. - Control of Cellular Aging, Tissue Function, and Cancer by p53 Downstream of Telomeres.
Roake CM, Artandi SE. Roake CM, et al. Cold Spring Harb Perspect Med. 2017 May 1;7(5):a026088. doi: 10.1101/cshperspect.a026088. Cold Spring Harb Perspect Med. 2017. PMID: 28289249 Free PMC article. Review. - DNA repair factors and telomere-chromosome integrity in mammalian cells.
Hande MP. Hande MP. Cytogenet Genome Res. 2004;104(1-4):116-22. doi: 10.1159/000077475. Cytogenet Genome Res. 2004. PMID: 15162024 Review.
Cited by
- Vascular Senescence: A Potential Bridge Between Physiological Aging and Neurogenic Decline.
Rojas-Vázquez S, Blasco-Chamarro L, López-Fabuel I, Martínez-Máñez R, Fariñas I. Rojas-Vázquez S, et al. Front Neurosci. 2021 Apr 20;15:666881. doi: 10.3389/fnins.2021.666881. eCollection 2021. Front Neurosci. 2021. PMID: 33958987 Free PMC article. Review. - Overexpression of the mTERT gene by adenoviral vectors promotes the proliferation of neuronal stem cells in vitro and stimulates neurogenesis in the hippocampus of mice.
Liu M, Hu Y, Zhu L, Chen C, Zhang Y, Sun W, Zhou Q. Liu M, et al. J Biomed Res. 2012 Sep;26(5):381-8. doi: 10.7555/JBR.26.20110078. Epub 2012 Apr 18. J Biomed Res. 2012. PMID: 23554775 Free PMC article. - Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls.
Kananen L, Surakka I, Pirkola S, Suvisaari J, Lönnqvist J, Peltonen L, Ripatti S, Hovatta I. Kananen L, et al. PLoS One. 2010 May 25;5(5):e10826. doi: 10.1371/journal.pone.0010826. PLoS One. 2010. PMID: 20520834 Free PMC article. - Stimulation of cell proliferation by glutathione monoethyl ester in aged bone marrow stromal cells is associated with the assistance of TERT gene expression and telomerase activity.
Aminizadeh N, Tiraihi T, Mesbah-Namin SA, Taheri T. Aminizadeh N, et al. In Vitro Cell Dev Biol Anim. 2016 Aug;52(7):772-81. doi: 10.1007/s11626-016-0021-5. Epub 2016 Jun 1. In Vitro Cell Dev Biol Anim. 2016. PMID: 27251157 - Telomere attrition is associated with declines in medial temporal lobe volume and white matter microstructure in functionally independent older adults.
Staffaroni AM, Tosun D, Lin J, Elahi FM, Casaletto KB, Wynn MJ, Patel N, Neuhaus J, Walters SM, Epel ES, Blackburn EH, Kramer JH. Staffaroni AM, et al. Neurobiol Aging. 2018 Sep;69:68-75. doi: 10.1016/j.neurobiolaging.2018.04.021. Epub 2018 May 8. Neurobiol Aging. 2018. PMID: 29859365 Free PMC article.
References
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
- Armesilla-Diaz A, Bragado P, Del Valle I, Cuevas E, Lazaro I, Martin C, Cigudosa JC, Silva A. p53 regulates the self-renewal and differentiation of neural precursors. Neuroscience. 2009;158:1378–1389. - PubMed
- Blasco MA. Telomerase beyond telomeres. Nat Rev Cancer. 2002;2:627–633. - PubMed
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous