CD40 ligand deficiency: neurologic sequelae with radiographic correlation - PubMed (original) (raw)
Case Reports
CD40 ligand deficiency: neurologic sequelae with radiographic correlation
Shrinivas Bishu et al. Pediatr Neurol. 2009 Dec.
Abstract
Patients with CD40 ligand deficiency are susceptible to central nervous system infections, but to date the neurologic progression or long-term outcome of central nervous system complications have not been reported in detail. Characterizing the central nervous system complications of immune deficiencies can lead to the identification of new pathogens. For this study, clinical data were reviewed on patients with both CD40 ligand deficiency and neurodegeneration, identified from a larger cohort of 31 patients. Five patients had progressive neurologic and cognitive decline in the absence of clinical signs of acute fulminant encephalitis, with anatomic brain abnormalities and high mortality (60%). Despite multiple evaluations, no pathogens were identified in four patients, all of whom were on standard intravenous immunoglobulin therapy at illness presentation. This clinical phenotype of progressive decline without acute fulminant encephalitis is similar to chronic enteroviral encephalitis in X-linked agammaglobulinemia, another condition with severe humoral immune defects. Whether infection secondary to subtherapeutic levels of central nervous system immunoglobulin G (IgG), inadequately protective levels of serum IgG, or impaired CD40 ligand-dependent IgG-independent antiviral responses contributed remains undetermined. Emerging gene-chip techniques applied in patients with primary immune deficiencies may identify heretofore unknown viruses. Prospective neurocognitive and evaluation of patients with CD40 ligand deficiency may identify affected patients before overt clinical signs appear.
Conflict of interest statement
Conflict of interest: No conflicts reported by any author
Figures
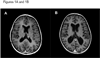
Figure 1. Radiographic findings of patients with CD40 ligand deficiency and neurodegeneration
(A) T1 weighted brain MRI obtained upon presentation in patient 1 and (B) three months later demonstrating progressive cortical atrophy. TR/TE: 400/10 ms, field of view: 20–22 cm, matrix: 256 × 192, 1 excitation, 5 mm slice thickness. (C) Fluid inversion recovery attenuation weighted MRI obtained in patient 2 at presentation. (D) Patient 2 three years later (one month after completing pleconaril therapy) demonstrating progressive atrophy and periventricular encephalomalacia (arrows). TR/TE: 10,000/145 ms, field of view: 20–22 cm, matrix: 256 × 192, 1 excitation, 5 mm slice thickness. (E) T1 weighted MRI obtained in patient 4 when 6 years after presenting with neurologic decline and then (F) 4 years later demonstrating progressive atrophy (interval gyral thining/sulcul widening (arrows)). TR/TE: 400/10 ms, field of view: 20–22 cm, matrix: 256 × 192, 1 excitation, 5 mm slice thickness.
Figure 1. Radiographic findings of patients with CD40 ligand deficiency and neurodegeneration
(A) T1 weighted brain MRI obtained upon presentation in patient 1 and (B) three months later demonstrating progressive cortical atrophy. TR/TE: 400/10 ms, field of view: 20–22 cm, matrix: 256 × 192, 1 excitation, 5 mm slice thickness. (C) Fluid inversion recovery attenuation weighted MRI obtained in patient 2 at presentation. (D) Patient 2 three years later (one month after completing pleconaril therapy) demonstrating progressive atrophy and periventricular encephalomalacia (arrows). TR/TE: 10,000/145 ms, field of view: 20–22 cm, matrix: 256 × 192, 1 excitation, 5 mm slice thickness. (E) T1 weighted MRI obtained in patient 4 when 6 years after presenting with neurologic decline and then (F) 4 years later demonstrating progressive atrophy (interval gyral thining/sulcul widening (arrows)). TR/TE: 400/10 ms, field of view: 20–22 cm, matrix: 256 × 192, 1 excitation, 5 mm slice thickness.
Figure 1. Radiographic findings of patients with CD40 ligand deficiency and neurodegeneration
(A) T1 weighted brain MRI obtained upon presentation in patient 1 and (B) three months later demonstrating progressive cortical atrophy. TR/TE: 400/10 ms, field of view: 20–22 cm, matrix: 256 × 192, 1 excitation, 5 mm slice thickness. (C) Fluid inversion recovery attenuation weighted MRI obtained in patient 2 at presentation. (D) Patient 2 three years later (one month after completing pleconaril therapy) demonstrating progressive atrophy and periventricular encephalomalacia (arrows). TR/TE: 10,000/145 ms, field of view: 20–22 cm, matrix: 256 × 192, 1 excitation, 5 mm slice thickness. (E) T1 weighted MRI obtained in patient 4 when 6 years after presenting with neurologic decline and then (F) 4 years later demonstrating progressive atrophy (interval gyral thining/sulcul widening (arrows)). TR/TE: 400/10 ms, field of view: 20–22 cm, matrix: 256 × 192, 1 excitation, 5 mm slice thickness.
Similar articles
- CD40 Ligand Deficiency.
Leite LFB, Máximo TA, Mosca T, Forte WCN. Leite LFB, et al. Allergol Immunopathol (Madr). 2020 Jul-Aug;48(4):409-413. doi: 10.1016/j.aller.2019.08.005. Epub 2019 Dec 9. Allergol Immunopathol (Madr). 2020. PMID: 31831191 Review. - Hematopoietic Stem Cell Transplantation for CD40 Ligand Deficiency: Single Institution Experience.
Allewelt H, Martin PL, Szabolcs P, Chao N, Buckley R, Parikh S. Allewelt H, et al. Pediatr Blood Cancer. 2015 Dec;62(12):2216-22. doi: 10.1002/pbc.25711. Epub 2015 Aug 20. Pediatr Blood Cancer. 2015. PMID: 26291959 Clinical Trial. - X-linked hyper-IgM syndrome associated with a rapid course of multifocal leukoencephalopathy.
Aschermann Z, Gomori E, Kovacs GG, Pal E, Simon G, Komoly S, Marodi L, Illes Z. Aschermann Z, et al. Arch Neurol. 2007 Feb;64(2):273-6. doi: 10.1001/archneur.64.2.273. Arch Neurol. 2007. PMID: 17296845 - Immunoglobulin class switch recombination deficiency type 1 or CD40 ligand deficiency: from bedside to bench and back again.
Hirbod-Mobarakeh A, Aghamohammadi A, Rezaei N. Hirbod-Mobarakeh A, et al. Expert Rev Clin Immunol. 2014 Jan;10(1):91-105. doi: 10.1586/1744666X.2014.864554. Epub 2013 Nov 26. Expert Rev Clin Immunol. 2014. PMID: 24308834 Review. - CD40 ligand deficiency: treatment strategies and novel therapeutic perspectives.
França TT, Barreiros LA, Al-Ramadi BK, Ochs HD, Cabral-Marques O, Condino-Neto A. França TT, et al. Expert Rev Clin Immunol. 2019 May;15(5):529-540. doi: 10.1080/1744666X.2019.1573674. Epub 2019 Feb 18. Expert Rev Clin Immunol. 2019. PMID: 30681380 Review.
Cited by
- Distinct plasma immune signatures in ME/CFS are present early in the course of illness.
Hornig M, Montoya JG, Klimas NG, Levine S, Felsenstein D, Bateman L, Peterson DL, Gottschalk CG, Schultz AF, Che X, Eddy ML, Komaroff AL, Lipkin WI. Hornig M, et al. Sci Adv. 2015 Feb;1(1):e1400121. doi: 10.1126/sciadv.1400121. Sci Adv. 2015. PMID: 26079000 Free PMC article. - Infections in Inborn Errors of Immunity with Combined Immune Deficiency: A Review.
George K, Govindaraj G. George K, et al. Pathogens. 2023 Feb 7;12(2):272. doi: 10.3390/pathogens12020272. Pathogens. 2023. PMID: 36839544 Free PMC article. Review. - The role of tumor necrosis factor receptor superfamily members in mammalian brain development, function and homeostasis.
Twohig JP, Cuff SM, Yong AA, Wang EC. Twohig JP, et al. Rev Neurosci. 2011;22(5):509-33. doi: 10.1515/RNS.2011.041. Epub 2011 Aug 24. Rev Neurosci. 2011. PMID: 21861782 Free PMC article. Review. - Downregulation of CD40L-CD40 attenuates seizure susceptibility and severity of seizures.
Pototskiy E, Vinokuroff K, Ojeda A, Major CK, Sharma D, Anderson T, Howard K, Borenstein R, Musto AE. Pototskiy E, et al. Sci Rep. 2021 Aug 26;11(1):17262. doi: 10.1038/s41598-021-96760-3. Sci Rep. 2021. PMID: 34446808 Free PMC article. - Cryptococcal Meningitis and Post-Infectious Inflammatory Response Syndrome in a Patient With X-Linked Hyper IgM Syndrome: A Case Report and Review of the Literature.
Romani L, Williamson PR, Di Cesare S, Di Matteo G, De Luca M, Carsetti R, Figà-Talamanca L, Cancrini C, Rossi P, Finocchi A. Romani L, et al. Front Immunol. 2021 Jul 15;12:708837. doi: 10.3389/fimmu.2021.708837. eCollection 2021. Front Immunol. 2021. PMID: 34335625 Free PMC article. Review.
References
- Seyama K, Nonoyama S, Gangsaas I, Hollenbaugh D, Pabst HF, Aruffo A, Ochs HD. Mutations of the CD40 ligand gene and its effect on CD40 ligand expression in patients with X-linked hyper IgM syndrome. Blood. 1998;92:2421–2434. - PubMed
- Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6550–6554. - PMC - PubMed
- Lane P, Traunecker A, Hubele S, Inui S, Lanzavecchia A, Gray D. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. European journal of immunology. 1992;22:2573–2578. - PubMed
- Levy J, Espanol-Boren T, Thomas C, Fischer A, Tovo P, Bordigoni P, Resnick I, Fasth A, Baer M, Gomez L, Sanders EA, Tabone MD, Plantaz D, Etzioni A, Monafo V, Abinun M, Hammarstrom L, Abrahamsen T, Jones A, Finn A, Klemola T, DeVries E, Sanal O, Peitsch MC, Notarangelo LD. Clinical spectrum of X-linked hyper-IgM syndrome. J Pediatr. 1997;131:47–54. - PubMed
- Cunningham CK, Bonville CA, Ochs HD, Seyama K, John PA, Rotbart HA, Weiner LB. Enteroviral meningoencephalitis as a complication of X-linked hyper IgM syndrome. The Journal of pediatrics. 1999;134:584–588. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials