Insulin sensitizing pharmacology of thiazolidinediones correlates with mitochondrial gene expression rather than activation of PPAR gamma - PubMed (original) (raw)
Patrick M Blanner, William G McDonald, Nicholas R Staten, Richard A Mazzarella, Graciela B Arhancet, Martin F Meier, David J Weiss, Patrick M Sullivan, Alexander E Hromockyj, Rolf F Kletzien, Jerry R Colca
Affiliations
- PMID: 19936080
- PMCID: PMC2759129
Insulin sensitizing pharmacology of thiazolidinediones correlates with mitochondrial gene expression rather than activation of PPAR gamma
Charles W Bolten et al. Gene Regul Syst Bio. 2007.
Abstract
Insulin sensitizing thiazolidinediones (TZDs) are generally considered to work as agonists for the nuclear receptor peroxisome proliferative activated receptor-gamma (PPAR gamma). However, TZDs also have acute, non-genomic metabolic effects and it is unclear which actions are responsible for the beneficial pharmacology of these compounds. We have taken advantage of an analog, based on the metabolism of pioglitazone, which has much reduced ability to activate PPAR gamma. This analog (PNU-91325) was compared to rosiglitazone, the most potent PPAR gamma activator approved for human use, in a variety of studies both in vitro and in vivo. The data demonstrate that PNU-91325 is indeed much less effective than rosiglitazone at activating PPAR gamma both in vitro and in vivo. In contrast, both compounds bound similarly to a mitochondrial binding site and acutely activated PI-3 kinase-directed phosphorylation of AKT, an action that was not affected by elimination of PPAR gamma activation. The two compounds were then compared in vivo in both normal C57 mice and diabetic KKAy mice to determine whether their pharmacology correlated with biomarkers of PPAR gamma activation or with the expression of other gene transcripts. As expected from previous studies, both compounds improved insulin sensitivity in the diabetic mice, and this occurred in spite of the fact that there was little increase in expression of the classic PPAR gamma target biomarker adipocyte binding protein-2 (aP2) with PNU-91325 under these conditions. An examination of transcriptional profiling of key target tissues from mice treated for one week with both compounds demonstrated that the relative pharmacology of the two thiazolidinediones correlated best with an increased expression of an array of mitochondrial proteins and with expression of PPAR gamma coactivator 1-alpha (PGC1 alpha), the master regulator of mitochondrial biogenesis. Thus, important pharmacology of the insulin sensitizing TZDs may involve acute actions, perhaps on the mitochondria, that are independent of direct activation of the nuclear receptor PPAR gamma. These findings suggest a potential alternative route to the discovery of novel insulin sensitizing drugs.
Keywords: diabetes; insulin sensitizer; mechanism of action; mitoNEET; mitochondria; thiazolidinedione.
Figures
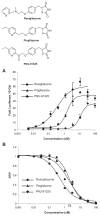
Figure 1
PNU-91325, pioglitazone and rosiglitazone activation of Gal4-PPARγ versus mitochondrial binding. The structures of the thiazolidinediones used in these studies are shown (Top to bottom: rosiglitazone, pioglitazone and PNU-91325). A. (upper panel) HUH7 cells expressing Gal4-PPARγ were treated with doses of thiazolidinediones shown on the abscissa and the luciferase response is recorded on the ordinate as discussed in the text. The data are an average and SEM of triplicate experiments with this cell type. *p < 0.05 versus rosiglitazone at the same concentration. Similar data were obtained on other occasions with CHO and HepG2 cells (not shown). B. (lower panel) Solubilized liver mitochondrial membranes were incubated with 3H-pioglitazone in the combined presence of the concentration of the compounds shown on the abscissa and as discussed in the text. The data are plotted as the ratio of specific counts without competition to those in the presence of the indicated concentration of unlabeled competitor. These data are average of triplicate points and SEM (note: most SEMs fall within the symbols) of a single experiment, but are representative of more than three experiments with liver mitochondrial membranes.
Figure 2
Direct increase in p-AKT in vitro is not dependent on PPARγ activation. HUH7 cells were treated and phosphorylated AKT was measured and expressed as a ratio to total AKT as described in the text. A. Shows the time course of increased content of pAKT with rosiglitazone (open symbols) and PNU-91325 (closed symbols). The time of exposure to the TZDs is shown on the abscissa and the ratio of the increase in pAKT/total AKT is shown on the ordinate. Data are mean and SEM of triplicate determinations. B. Rosiglitazone activation of AKT is not blocked by a PPARγ antagonist. Wells were pretreated for 1 hour with (solid symbols) or without (open symbols) the PPARγ antagonist T0070907 (10 μM) and then treated with the concentrations of rosiglitazone shown on the abscissa for 4 hours. Half of the wells were also treated with half-maximal insulin (1 nM; dotted lines) 5 minutes before harvesting the cells for measurement of the content of phosphorylated AKT as described in the text. The data are the mean and SEM of triplicate wells from a representative experiment. C. PNU-91325 activation of AKT is not blocked by a PPARγ antagonist. The same experimental protocol and data presentation as in Panel B except that the TZD used was PNU-91325. D. Activation of AKT by TZDs is blocked by wortmannin. The experimental protocol and data presentation are the same as those described in Panel A except that wortmannin was added 1 hour before rosiglitazone or PNU-91325.
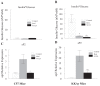
Figure 3
Improvement of insulin sensitivity as compared to activation of the PPARγ biomarker, aP2, produced by PNU-91325 and rosiglitazone. C57 normal mice and diabetic KKAy mice were treated orally with a single maximally effective dose of rosiglitazone (20 mg/kg), PNU-91325 (100 mg/kg) or vehicle for 7 days. On the 8th day and after a 4 hour fast, circulating glucose and insulin levels were measured. Fasting levels of glucose and insulin averaged 22.7 mM and 3.7 nM, respectively for the diabetic KKAy mice and 12 mM and 0.1 nM, respectively for the non-diabetic C57 mice in the control (vehicle) groups. The product of insulin (nM) and glucose (mM) is presented as an index of any changes in insulin sensitivity. This statistic is shown for the normal mice in Panel A and for the diabetic KKAy mice in Panel B (mean and SEM; n = 8). The relative expression of the PPARγ biomarker aP2 in the liver of C57 mice (mean and SEM; n = 8) is shown in Panel C and the relative expression of aP2 in the liver of KKAy mice (mean and SEM; n = 8) is shown in Panel D. As expected, there was not a measurable change in insulin sensitivity in non-diabetic mice. Whereas this dose of PNU-91325 produced a similar increase in insulin sensitivity as did rosiglitazone (decrease in glucose*insulin), it produced less increase in the expression of the aP2 transcript. *p < 0.05 versus vehicle. # p < 0.05 versus rosiglitazone.
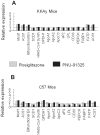
Figure 4
Comparison of regulation of PPRE-regulated genes in adipose tissue from normal and diabetic mice. Data for genes containing PPRE (selected as discussed in the text) were taken from the transcriptional profiling of epididymal fat pads of KKAy (A) and C57 mice (B) shown in Figure 4. The respective transcript levels were compared for the gene shown on the abscissa in the samples from rosiglitazone-treated mice (gray) and PNU-91325-treated mice (black) versus vehicle (taken as 1). Data are from all 8 mice of each group. Malic enzyme, supernatant (Mod1), [NM_008615]; Acyl-CoA synthetase long-chain family member 1 (Acsl1), [NM_007981]; Enoyl-Coenzyme A, hydratase/3-hydroxyacyl Coenzyme A dehydrogenase (Ehhadh), [NM_023737]; Stearoyl-Coenzyme A desaturase 1 [7530417E14]; 3-hydroxy-3-methylglutaryl-coenzyme A synthase 1. [NM_145942]; Glycerol phosphate dehydrogenase 1, [ 0610008N20]; Apolipoprotein A-I (Apoa1), [NM_009692]; Apolipoprotein A-II (Apoa2), [NM_013474]; Apolipoprotein C-III (Apoc3), [NM_023114]; Fatty acid binding protein 4, adipocyte (Fabp4), [NM_024406]; Lipoprotein lipase (Lpl), [NM_008509]; CD36 antigen (Cd36), [NM_007643]; Phosphoenolpyruvate carboxykinase 1, cytosolic (Pck1), [NM_011044]; Fatty acid binding protein 1, liver (Fabp1), [NM_017399]; Solute carrier family 27 (fatty acid transporter), member 1 [NM_011977] (FATP); Acyl-CoA synthetase long-chain family member 1 (Acsl1), [NM_ 007981].
Figure 5
Comparison of the effects of PNU-91325 and rosiglitazone treatment on transcripts for mitochondrial proteins. Data were extracted from the transcriptional profile of >300 mitochondrial proteins from the array data from the mice shown in Figure 3 and 4 and relative increase with each thiazolidinedione versus vehicle were plotted. For each of the transcripts, the relative increase produced by PNU-91325 versus vehicle is plotted on the ordinate as compared to the relative increase in the same transcript produced by rosiglitazone on the abscissa. On average, the effect of PNU-91325 was 1.4 times that of rosiglitazone.
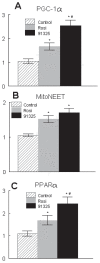
Figure 6
Effects of PNU-91325 and rosiglitazone on expression of PGC1α, mitoNEET and PPARα. RNA was isolated from the epididymal fat pads of diabetic KKAy mice treated with vehicle (lined), rosiglitazone (gray) or PNU-91325 (black) bars. The expression of transcripts relative to vehicle was determined for PGC1α (A), mitoNEET (B) and PPARα (C) (data are mean and SEM; n = 8). *p < 0.05 versus vehicle. # p < 0.05 versus rosiglitazone.
Similar articles
- The beneficial metabolic effects of insulin sensitizers are not attenuated by mitochondrial pyruvate carrier 2 hypomorphism.
Vigueira PA, McCommis KS, Hodges WT, Schweitzer GG, Cole SL, Oonthonpan L, Taylor EB, McDonald WG, Kletzien RF, Colca JR, Finck BN. Vigueira PA, et al. Exp Physiol. 2017 Aug 1;102(8):985-999. doi: 10.1113/EP086380. Epub 2017 Jul 10. Exp Physiol. 2017. PMID: 28597936 Free PMC article. - Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key?
Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, Dello Russo C. Feinstein DL, et al. Biochem Pharmacol. 2005 Jul 15;70(2):177-88. doi: 10.1016/j.bcp.2005.03.033. Biochem Pharmacol. 2005. PMID: 15925327 Review. - Thiazolidinedione activation of peroxisome proliferator-activated receptor gamma can enhance mitochondrial potential and promote cell survival.
Wang YL, Frauwirth KA, Rangwala SM, Lazar MA, Thompson CB. Wang YL, et al. J Biol Chem. 2002 Aug 30;277(35):31781-8. doi: 10.1074/jbc.M204279200. Epub 2002 Jun 24. J Biol Chem. 2002. PMID: 12082115 - Experimental approaches to study PPAR gamma agonists as antidiabetic drugs.
Vázquez M, Silvestre JS, Prous JR. Vázquez M, et al. Methods Find Exp Clin Pharmacol. 2002 Oct;24(8):515-23. doi: 10.1358/mf.2002.24.8.705072. Methods Find Exp Clin Pharmacol. 2002. PMID: 12500431 Review.
Cited by
- Novel insulin sensitizer modulates nutrient sensing pathways and maintains β-cell phenotype in human islets.
Rohatgi N, Aly H, Marshall CA, McDonald WG, Kletzien RF, Colca JR, McDaniel ML. Rohatgi N, et al. PLoS One. 2013 May 1;8(5):e62012. doi: 10.1371/journal.pone.0062012. Print 2013. PLoS One. 2013. PMID: 23650507 Free PMC article. - Development of Therapeutics That Induce Mitochondrial Biogenesis for the Treatment of Acute and Chronic Degenerative Diseases.
Cameron RB, Beeson CC, Schnellmann RG. Cameron RB, et al. J Med Chem. 2016 Dec 8;59(23):10411-10434. doi: 10.1021/acs.jmedchem.6b00669. Epub 2016 Sep 27. J Med Chem. 2016. PMID: 27560192 Free PMC article. Review. - Pioglitazone and Deoxyribonucleoside Combination Treatment Increases Mitochondrial Respiratory Capacity in m.3243A>G MELAS Cybrid Cells.
Burgin HJ, Lopez Sanchez MIG, Smith CM, Trounce IA, McKenzie M. Burgin HJ, et al. Int J Mol Sci. 2020 Mar 20;21(6):2139. doi: 10.3390/ijms21062139. Int J Mol Sci. 2020. PMID: 32244971 Free PMC article. - Role and treatment of mitochondrial DNA-related mitochondrial dysfunction in sporadic neurodegenerative diseases.
Swerdlow RH. Swerdlow RH. Curr Pharm Des. 2011;17(31):3356-73. doi: 10.2174/138161211798072535. Curr Pharm Des. 2011. PMID: 21902672 Free PMC article. Review. - Rosiglitazone-induced mitochondrial biogenesis in white adipose tissue is independent of peroxisome proliferator-activated receptor γ coactivator-1α.
Pardo R, Enguix N, Lasheras J, Feliu JE, Kralli A, Villena JA. Pardo R, et al. PLoS One. 2011;6(11):e26989. doi: 10.1371/journal.pone.0026989. Epub 2011 Nov 7. PLoS One. 2011. PMID: 22087241 Free PMC article.
References
- Bogacka I, Xie H, Bray GA, et al. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54:1392–9. - PubMed
- Brunmair B, Gras F, Neschen S, et al. Direct thiazolidinedione action on isolated rat skeletal muscle fuel handling is independent of peroxisome proliferator-activated receptor-gamma-mediated changes in gene expression. Diabetes. 2001;50:2309–15. - PubMed
- Brunmair B, Staniek K, Gras F, et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53:1052–9. - PubMed
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. - PubMed
- Chawla A, Barak Y, Nagy L, et al. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous