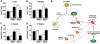GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice - PubMed (original) (raw)
GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice
Gang Li et al. Cell Stem Cell. 2009.
Abstract
Apolipoprotein (apo) E, a polymorphic protein with three isoforms (apoE2, apoE3, and apoE4), is essential for lipid homeostasis. Carriers of apoE4 are at higher risk for developing Alzheimer's disease. We have investigated adult neurogenesis in mice with knockout (KO) for apoE or with knockin (KI) alleles for human apoE3 or apoE4, and we report that neurogenesis is reduced in both apoE-KO and apoE4-KI mice. In apoE-KO mice, increased BMP signaling promoted glial differentiation at the expense of neurogenesis. In contrast, in apoE4-KI mice, presynaptic GABAergic input-mediated maturation of newborn neurons was diminished. Tau phosphorylation, an Alzheimer's disease characteristic, and levels of neurotoxic apoE fragments were both elevated in apoE4-KI hippocampal neurons concomitant with decreased GABAergic interneuron survival. Potentiating GABAergic signaling restored neuronal maturation and neurogenesis in apoE4-KI mice to normal levels. These findings suggest that GABAergic signaling can be targeted to mitigate the deleterious effects of apoE4 on neurogenesis.
Figures
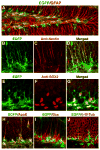
Figure 1. ApoE Is Expressed in Hippocampal NSCs
(A–J)Confocal images of the dentate gyrus of EGFPapoE reporter mice. Green indicates EGFP representing apoE (A, B, D, E, G–J). Red indicates immunostaining positive for anti-GFAP (A), anti-nestin (C, D), anti-Sox2 (F, G), anti-apoE (H), anti-Dcx (I), or anti-β-III-tubulin (J).
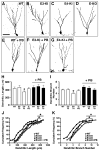
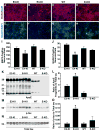
Figure 2. Effects of ApoE Deficiency and ApoE Isoforms on Hippocampal Neurogenesis and Astrogenesis
(A, B) Representative confocal images of the BrdU-positive cells in the SGZ of female apoE3-KI (A) and apoE4-KI (B) mice at 6–7 months of age were collected 1 day after BrdU injection. (C, D) Representative confocal images of the BrdU and NeuN double positive cells in the SGZ of female apoE3-KI (C) and apoE4-KI (D) mice at 6–7 months of age were collected 4 weeks after BrdU injection. (E, F) Representative confocal images of the BrdU and S100β double positive cells in the SGZ of female wildtype (E) and apoE-KO (F) mice at 6–7 months of age were collected 4 weeks after BrdU injection. (G–J) Numbers of newborn cells (BrdU+) (G), immature neurons (BrdU+/Dcx+) (H), mature neurons (BrdU+/NeuN+) (I), and astrocytes (BrdU+/S100β+) (J) in the SGZ of female mice of various apoE genotypes at 6–7 months of age were determined 1 and 3 days and 4 and 10 weeks after BrdU injection. Values are mean ± SD (n = 4–6 mice per genotype). * p < 0.05 vs. other groups (t test).M (K) Total numbers of Sox2-positive cells in the SGZ of female wildtype, apoE3-KI, apoE4-KI, and apoE-KO mice at 6–7 months of age. Values are mean ± SD (n = 4 mice per genotype).M (L) Numbers of BrdU and Sox2 double-positive cells in the SGZ of female wildtype, apoE3-KI, apoE4-KI, and apoE-KO mice at 6–7 months of age were determined 1 day after BrdU injection. Values are mean ± SD (n = 4 mice per genotype). (M) Total numbers of Ki67-positive cells in the SGZ of female wildtype, apoE3-KI, apoE4-KI, and apoE-KO mice at 6–7 months of age. Values are mean ± SD (n = 4 mice per genotype). (N–P) Numbers of immature neurons (BrdU+/Dcx+) (N), astrocytes (BrdU+/S100β+) (O), and mature neurons (BrdU+/NeuN+) (P) in the SGZ of female mice of various apoE genotypes at 6–7 months of age were determined at 3 days and 4 weeks after BrdU injection. Values are mean ± SD (n = 4–6 mice per genotype). * p < 0.05 vs. wildtype and apoE3-KI mice (t test).
Figure 3. ApoE4 Impairs Dendritic Development of Newborn Neurons in the Hippocampus
(A–G) Confocal three-dimensional reconstruction of dendrites (inverted images) of newborn neurons (4 weeks after retrovirus-GFP injection) in the dentate gyrus of wildtype (A), apoE3-KI (B), apoE4-KI (C), and apoE-KO (D) mice, wildtype mice treated with PB (E), apoE3-KI mice treated with PB (F), and apoE4-KI mice treated with PB (G). Scale bar, 50 µm. (H–K) Total dendritic length (H, J) and dendritic branch number (I, K) of newborn neurons. * p < 0.05 (t test in H and I; Kolmogorov-Smirnov test in J and K). WT, n = 43; E3-KI, n = 84; E4-KI, n = 73; E-KO, n =35; WT + PB, n =52; E3-KI + PB, n =42; E4-KI + PB, n = 31. Values in panels H and I are mean ± SEM.
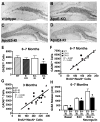
Figure 4. ApoE4 Impairs GABAergic Interneurons and GABA Release in the Hippocampus
(A–D) Immunostaining of GAD67-positive GABAergic interneurons in the hilus of female wildtype (A), apoE-KO (B), apoE3-KI (C), and apoE4-KI (D) mice at 6–7 months of age. (E) Numbers of GAD67-positive GABAergic interneurons in different mice at 6–7 months of age. Values are mean ± SD (n = 4–7 mice per genotype). * p < 0.05 vs. wildtype and apoE3-KI mice (t test). (F, G) Positive correlation between the number of GAD67-positive interneurons and the number of BrdU+/NeuN+ neurons among female wildtype, apoE3-KI, and apoE4-KI mice at 6–7 months of age (F, n = 12 mice) and at 3 months of age (G, n = 15 mice). (H) GABA release in hippocampal slices, determined by mass spectrometry. Values are mean ± SD (n = 4–7 mice per genotype). * p < 0.05 vs. wildtype and apoE3-KI mice (t test).
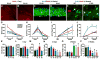
Figure 5. ApoE4 Generates More Neurotoxic Fragments, Increases Tau Phosphorylation, and Decreases GABAergic Neuron Survival in Primary Hippocampal Neuronal Cultures
(A–H) Primary hippocampal neuron cultures were prepared from P0 pups of apoE3-KI, apoE4-KI, wildtype, and apoE-KO mice, cultured for 14 days in vitro (14 DIV), and stained with anti-MAP2 (red) and DAPI (blue) (A–D) or anti-GAD67 (green) and DAPI (blue) (E–H). Shown are representative images from five coverslips of each genotype and five fields per coverslip (magnification, 200x). (I, J) MAP2-positive (I) and GAD67-positive (J) neurons were quantified. Values are mean ± SEM (five images per coverslip and five coverslips per genotype). * p < 0.05 versus other groups (t test). (K) Anti-apoE western blot of primary neuron lysates from apoE3-KI, apoE4-KI, wildtype, and apoE-KO mice. Note that mouse apoE is 5 amino acids shorter than human apoE. (L) ApoE fragmentation, reported as the ratio of total apoE fragments to total tau. Values are mean ± SD (n = 3–4 mice per genotype). * p < 0.001 versus other groups (t test). (M, N) Anti-p-tau (M, AT8 monoclonal antibody) and anti-total tau (N, tau-5 monoclonal antibody) western blots of primary neuron lysates from apoE3-KI, apoE4-KI, wildtype, and apoE-KO mice. (O) The level of tau phosphorylation, reported as the ratio of p-tau to total tau. Values are mean ± SD (n = 3–4 mice per genotype). * p < 0.001 versus other groups (t test).
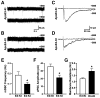
Figure 6. ApoE4 Impairs the GABAergic Electrophysiological Inputs in Newborn Neurons in the Hippocampus
(A, B) Sample traces of mSSCs in a GFP+ neuron 2 weeks after retrovirus-GFP injection from an apoE3-KI (A) or an apoE4-KI (B) mouse during whole-cell voltage clamp recording in the presence of DNQX (20 μM), D-AP5 (50 μM), and TTX (1 μM). The mSSCs were blocked by bath application of BMI (100 μM). Scale bars, 10 pA and 5 s. (C, D) Sample traces of ePSCs in a GFP+ neuron at 2 weeks after retrovirus-GFP injection from an apoE3-KI (C) or an apoE4-KI (D) mouse during whole-cell voltage clamp recording in the presence of DNQX (20 μM) and D-AP5 (50 μM). Currents were blocked by bath application of BMI (100 μM). Scale bars: 10 pA and 50 ms. (E) Average mSSC frequency in GFP+ neurons was lower in apoE4-KI mice than apoE3-KI mice. Values are mean ± SD (n = 21–28 cells per genotype). * p < 0.05 vs. apoE3-KI mice (t test). (F) Average ePSC amplitude in GFP+ neurons was lower in apoE4-KI mice than in apoE3-KI mice. Values are mean ± SD (n = 21–28 cells per genotype). * p < 0.05 vs. apoE3-KI mice (t test). (G) Average membrane resistance of GFP+ neurons in apoE3-KI and apoE4-KI mice 2 weeks after retrovirus-GFP injection. Values are mean ± SD (n = 40 cells per genotype). * p < 0.005 vs. apoE3-KI mice (t test).
Figure 7. GABAA Receptor Potentiator Rescues ApoE4-Induced Impairment of Hippocampal Neurogenesis and A Working Model for the Roles of ApoE and Its Isoforms in Adult Hippocampal Neurogenesis
Female apoE3-KI and apoE4-KI mice at 6–7 months of age were treated with a GABAA receptor potentiator (PB, 50 mg/kg) or antagonist (PTX, 4 mg/kg) as described in the text. BrdU-positive cells in the SGZ were counted at 1 day (A) and 4 weeks (B), and immature neurons (C) and mature neurons (D) were counted at 4 weeks. Untreated wildtype mice at 6–7 months of age served as controls. Values are mean ± SD (n = 4–6 mice per genotype). * p < 0.01 vs. untreated mice of the same apoE genotype (t test). (E) A working model for the roles of apoE and its isoforms in adult hippocampal neurogenesis. Adult hippocampal NSCs express apoE, which plays an important role in cell fate determination of NSCs toward neuronal development. ApoE deficiency stimulates astrogenesis and inhibits neurogenesis. ApoE4 decreases hippocampal neurogenesis by inhibiting neuronal maturation of NSCs through impairing GABAergic input onto newborn neurons.
Comment in
- Preview. Adult neurogenesis is altered by GABAergic imbalance in models of Alzheimer's disease.
Schinder AF, Morgenstern NA. Schinder AF, et al. Cell Stem Cell. 2009 Dec 4;5(6):573-4. doi: 10.1016/j.stem.2009.11.007. Cell Stem Cell. 2009. PMID: 19951683
Similar articles
- Apolipoprotein E4 causes age- and sex-dependent impairments of hilar GABAergic interneurons and learning and memory deficits in mice.
Leung L, Andrews-Zwilling Y, Yoon SY, Jain S, Ring K, Dai J, Wang MM, Tong L, Walker D, Huang Y. Leung L, et al. PLoS One. 2012;7(12):e53569. doi: 10.1371/journal.pone.0053569. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300939 Free PMC article. - Differential Signaling Mediated by ApoE2, ApoE3, and ApoE4 in Human Neurons Parallels Alzheimer's Disease Risk.
Huang YA, Zhou B, Nabet AM, Wernig M, Südhof TC. Huang YA, et al. J Neurosci. 2019 Sep 11;39(37):7408-7427. doi: 10.1523/JNEUROSCI.2994-18.2019. Epub 2019 Jul 22. J Neurosci. 2019. PMID: 31331998 Free PMC article. - Enhancing GABA Signaling during Middle Adulthood Prevents Age-Dependent GABAergic Interneuron Decline and Learning and Memory Deficits in ApoE4 Mice.
Tong LM, Yoon SY, Andrews-Zwilling Y, Yang A, Lin V, Lei H, Huang Y. Tong LM, et al. J Neurosci. 2016 Feb 17;36(7):2316-22. doi: 10.1523/JNEUROSCI.3815-15.2016. J Neurosci. 2016. PMID: 26888940 Free PMC article. - Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer's disease.
Butterfield DA, Mattson MP. Butterfield DA, et al. Neurobiol Dis. 2020 May;138:104795. doi: 10.1016/j.nbd.2020.104795. Epub 2020 Feb 6. Neurobiol Dis. 2020. PMID: 32036033 Free PMC article. Review. - Apolipoprotein E4, inhibitory network dysfunction, and Alzheimer's disease.
Najm R, Jones EA, Huang Y. Najm R, et al. Mol Neurodegener. 2019 Jun 11;14(1):24. doi: 10.1186/s13024-019-0324-6. Mol Neurodegener. 2019. PMID: 31186040 Free PMC article. Review.
Cited by
- Small molecule structure correctors abolish detrimental effects of apolipoprotein E4 in cultured neurons.
Chen HK, Liu Z, Meyer-Franke A, Brodbeck J, Miranda RD, McGuire JG, Pleiss MA, Ji ZS, Balestra ME, Walker DW, Xu Q, Jeong DE, Budamagunta MS, Voss JC, Freedman SB, Weisgraber KH, Huang Y, Mahley RW. Chen HK, et al. J Biol Chem. 2012 Feb 17;287(8):5253-66. doi: 10.1074/jbc.M111.276162. Epub 2011 Dec 12. J Biol Chem. 2012. PMID: 22158868 Free PMC article. - Comprehensive characterization of the neurogenic and neuroprotective action of a novel TrkB agonist using mouse and human stem cell models of Alzheimer's disease.
Charou D, Rogdakis T, Latorrata A, Valcarcel M, Papadogiannis V, Athanasiou C, Tsengenes A, Papadopoulou MA, Lypitkas D, Lavigne MD, Katsila T, Wade RC, Cader MZ, Calogeropoulou T, Gravanis A, Charalampopoulos I. Charou D, et al. Stem Cell Res Ther. 2024 Jul 6;15(1):200. doi: 10.1186/s13287-024-03818-w. Stem Cell Res Ther. 2024. PMID: 38971770 Free PMC article. - Transcranial Magnetic Stimulation in Alzheimer's Disease: Are We Ready?
Weiler M, Stieger KC, Long JM, Rapp PR. Weiler M, et al. eNeuro. 2020 Jan 7;7(1):ENEURO.0235-19.2019. doi: 10.1523/ENEURO.0235-19.2019. Print 2020 Jan/Feb. eNeuro. 2020. PMID: 31848209 Free PMC article. Review. - Pathogenesis of peritumoral hyperexcitability in an immunocompetent CRISPR-based glioblastoma model.
Hatcher A, Yu K, Meyer J, Aiba I, Deneen B, Noebels JL. Hatcher A, et al. J Clin Invest. 2020 May 1;130(5):2286-2300. doi: 10.1172/JCI133316. J Clin Invest. 2020. PMID: 32250339 Free PMC article. - D-serine increases adult hippocampal neurogenesis.
Sultan S, Gebara EG, Moullec K, Toni N. Sultan S, et al. Front Neurosci. 2013 Aug 29;7:155. doi: 10.3389/fnins.2013.00155. eCollection 2013. Front Neurosci. 2013. PMID: 24009551 Free PMC article.
References
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. - PubMed
- Altman J, Dasq GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965:319–336. - PubMed
- Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
- Aoki K, Uchihara T, Sanjo N, Nakamura A, Ikeda K, Tsuchiya K, Wakayama Y. Increased expression of neuronal apolipoprotein E in human brain with cerebral infarction. Stroke. 2003;34:875–880. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous