Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond - PubMed (original) (raw)
Review
Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond
Hristelina Ilieva et al. J Cell Biol. 2009.
Abstract
Selective degeneration and death of one or more classes of neurons is the defining feature of human neurodegenerative disease. Although traditionally viewed as diseases mainly affecting the most vulnerable neurons, in most instances of inherited disease the causative genes are widely-usually ubiquitously-expressed. Focusing on amyotrophic lateral sclerosis (ALS), especially disease caused by dominant mutations in Cu/Zn superoxide dismutase (SOD1), we review here the evidence that it is the convergence of damage developed within multiple cell types, including within neighboring nonneuronal supporting cells, which is crucial to neuronal dysfunction. Damage to a specific set of key partner cells as well as to vulnerable neurons may account for the selective susceptibility of neuronal subtypes in many human neurodegenerative diseases, including Huntington's disease (HD), Parkinson's disease (PD), prion disease, the spinal cerebellar ataxias (SCAs), and Alzheimer's disease (AD).
Figures
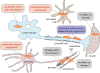
Figure 1.
Proposed mechanisms of toxicity in SOD1-mediated ALS. (A) Excitotoxicity is the hyperactivation of motor neurons resulting from failure to rapidly remove neurotransmitter glutamate from synapses due to deficiency in the glutamate transporter EAAT2 in the neighboring astrocytes. (B) ER stress is induced by abnormal interactions of mutant SOD1 with ER proteins (see text for details). (C) Proteasome inhibition due to “overload” of the proteasome degradation pathway with ubiquitinated misfolded protein aggregates may damage astrocytes and motor neurons. (D) Mitochondrial dysfunction mediated by mutant SOD1 deposition on the mitochondrial membrane provokes release of cytochrome c in motor neurons, whereas in astrocytes it leads to nitroxidative stress. (E) Toxic extracellular mutant SOD1 is secreted from motor neurons and astrocytes (not depicted) after interaction with components of neurosecretory vesicles. (F) Superoxide production from microglia or astrocytes can damage neighboring motor neurons. (G) Altered axonal transport including an increase in retrogradely transported stress-related proteins was reported in mutant SOD1-expressing motor neurons. (H) Synaptic vesicle defects such as stalling and loss from distal synapse in vulnerable motor neurons is an early event in ALS. (I) Loss of tight junction proteins within capillary endothelial cells results in the disruption of the blood–spinal cord barrier and the occurrence of microhemorrhages within the spinal cord well before disease onset.
Figure 2.
Contribution of mutant SOD1 within different cell types in ALS. Despite the apparent selectivity for motor neurons, multiple lines of evidence indicate that nonneuronal cell types contribute to pathogenesis and disease progression in SOD1-mediated neurodegeneration. Mutant SOD1 expression in motor neurons directs the onset and development of early disease, but does not influence its progression. In contrast, mutant SOD1 expression in microglia or astrocytes accelerates disease progression without affecting its onset. Expression of a dismutase-active mutant SOD1 specifically in Schwann cells was found to slow disease progression, but the role of a dismutase-inactive mutant in these cells has not been tested. Mutant SOD1 expression within muscle or endothelial cells does not affect ALS onset or progression, although some reports suggest that muscle might be a direct target of mutant SOD1 toxicity. Lastly, the vasculature is damaged very early in disease, leading to loss of tight junctions between endothelial cells and microhemorrhages, but whether any of this is from mutant SOD1 within pericytes, the terminal astrocyte, or coming from cells outside the vasculature is not established. 1(Ralph et al., 2005; Boillée et al., 2006; Jaarsma et al., 2008), 2(Beers et al., 2006; Boillée et al., 2006; Wang et al., 2009), 3(Yamanaka et al., 2008b), 4(Lobsiger et al., 2009), 5(Holzbaur et al., 2006; Miller et al., 2006; Dobrowolny et al., 2008; Towne et al., 2008), 6(Zhong et al., 2009).
Figure 3.
Non–cell autonomous pathogenesis in neurodegenerative diseases. This figure summarizes current evidence suggesting the contribution of apparently unaffected cell types in pathogenic mechanisms of neurodegenerative diseases, other than ALS. 1(Choi et al., 2008; Streit et al., 2009), 2(Nakamura et al., 1990; Ekblom et al., 1993), 3(Liberatore et al., 1999), 4(Yazawa et al., 2005), 5(Shin et al., 2005), 6(Sapp et al., 2001), 7(Custer et al., 2006), 8(Raeber et al., 1997; Jeffrey et al., 2004), 9(Falsig et al., 2008), 10(Prinz et al., 2004).
Similar articles
- Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis.
Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Yamanaka K, et al. Nat Neurosci. 2008 Mar;11(3):251-3. doi: 10.1038/nn2047. Epub 2008 Feb 3. Nat Neurosci. 2008. PMID: 18246065 Free PMC article. - [Superoxide dismutase-1 (SOD-1) gene mutation-dependent mechanisms of neural degeneration in amyotrophic lateral sclerosis].
Iłzecka J. Iłzecka J. Neurol Neurochir Pol. 2001 Mar-Apr;35(3):461-9. Neurol Neurochir Pol. 2001. PMID: 11732268 Review. Polish. - Ablation of proliferating microglia does not affect motor neuron degeneration in amyotrophic lateral sclerosis caused by mutant superoxide dismutase.
Gowing G, Philips T, Van Wijmeersch B, Audet JN, Dewil M, Van Den Bosch L, Billiau AD, Robberecht W, Julien JP. Gowing G, et al. J Neurosci. 2008 Oct 8;28(41):10234-44. doi: 10.1523/JNEUROSCI.3494-08.2008. J Neurosci. 2008. PMID: 18842883 Free PMC article. - Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells.
Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Marchetto MC, et al. Cell Stem Cell. 2008 Dec 4;3(6):649-57. doi: 10.1016/j.stem.2008.10.001. Cell Stem Cell. 2008. PMID: 19041781 - ALS: a disease of motor neurons and their nonneuronal neighbors.
Boillée S, Vande Velde C, Cleveland DW. Boillée S, et al. Neuron. 2006 Oct 5;52(1):39-59. doi: 10.1016/j.neuron.2006.09.018. Neuron. 2006. PMID: 17015226 Review.
Cited by
- Editorial: Dysfunction and Repair of Neural Circuits for Motor Control.
Tosolini AP, Mentis GZ, Martin JH. Tosolini AP, et al. Front Mol Neurosci. 2021 Mar 22;14:669824. doi: 10.3389/fnmol.2021.669824. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33828459 Free PMC article. No abstract available. - Chitotriosidase, a biomarker of amyotrophic lateral sclerosis, accentuates neurodegeneration in spinal motor neurons through neuroinflammation.
Varghese AM, Ghosh M, Bhagat SK, Vijayalakshmi K, Preethish-Kumar V, Vengalil S, Chevula PC, Nashi S, Polavarapu K, Sharma M, Dhaliwal RS, Philip M, Nalini A, Alladi PA, Sathyaprabha TN, Raju TR. Varghese AM, et al. J Neuroinflammation. 2020 Aug 6;17(1):232. doi: 10.1186/s12974-020-01909-y. J Neuroinflammation. 2020. PMID: 32762702 Free PMC article. - Proteostatic imbalance and protein spreading in amyotrophic lateral sclerosis.
Cicardi ME, Marrone L, Azzouz M, Trotti D. Cicardi ME, et al. EMBO J. 2021 May 17;40(10):e106389. doi: 10.15252/embj.2020106389. Epub 2021 Mar 31. EMBO J. 2021. PMID: 33792056 Free PMC article. Review. - EGFR inhibitor erlotinib delays disease progression but does not extend survival in the SOD1 mouse model of ALS.
Le Pichon CE, Dominguez SL, Solanoy H, Ngu H, Lewin-Koh N, Chen M, Eastham-Anderson J, Watts R, Scearce-Levie K. Le Pichon CE, et al. PLoS One. 2013 Apr 26;8(4):e62342. doi: 10.1371/journal.pone.0062342. Print 2013. PLoS One. 2013. PMID: 23638043 Free PMC article. - Aberrant neuregulin 1 signaling in amyotrophic lateral sclerosis.
Song F, Chiang P, Wang J, Ravits J, Loeb JA. Song F, et al. J Neuropathol Exp Neurol. 2012 Feb;71(2):104-15. doi: 10.1097/NEN.0b013e3182423c43. J Neuropathol Exp Neurol. 2012. PMID: 22249457 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous