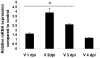Japanese encephalitis virus induce immuno-competency in neural stem/progenitor cells - PubMed (original) (raw)
Japanese encephalitis virus induce immuno-competency in neural stem/progenitor cells
Sulagna Das et al. PLoS One. 2009.
Abstract
Background: The low immunogenicity of neural stem/progenitor cells (NSPCs) coupled with negligible expression of MHC antigens has popularized their use in transplantation medicine. However, in an inflammatory environment, the NSPCs express costimulatory molecules and MHC antigens, and also exhibit certain immunomodulatory functions. Since NSPCs are the cellular targets in a number of virus infections both during postnatal and adult stages, we wanted to investigate the immunological properties of these stem cells in response to viral pathogen.
Methodology/principal findings: We utilized both in vivo mouse model and in vitro neurosphere model of Japanese encephalitis virus (JEV) infection for the study. The NSPCs residing in the subventricular zone of the infected brains showed prominent expression of MHC-I and costimulatory molecules CD40, CD80, and CD86. Using Flow cytometry and fluorescence microscopy, we observed increased surface expression of co-stimulatory molecule and MHC class I antigen in NSPCs upon progressive JEV infection in vitro. Moreover, significant production of pro-inflammatory cyto/chemokines was detected in JEV infected NSPCs by Cytokine Bead Array analysis. Interestingly, NSPCs were capable of providing functional costimulation to allogenic T cells and JEV infection resulted in increased proliferation of allogenic T cells, as detected by Mixed Lymphocyte reaction and CFSE experiments. We also report IL-2 production by NSPCs upon JEV infection, which possibly provides mitogenic signals to T cells and trigger their proliferation.
Conclusion/significance: The in vivo and in vitro findings clearly indicate the development of immunogenicity in NSPCs following progressive JEV infection, in our case, JEV infection. Following a neurotropic virus infection, NSPCs possibly behave as immunogenic cells and contribute to both the innate and adaptive immune axes. The newly discovered immunological properties of NSPCs may have implications in assigning a new role of these cells as non-professional antigen presenting cells in the central nervous system.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
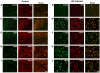
Figure 1. Upregulation of costimulatory molecules and MHC class I in Nestin-positive cells in SVZ during JEV infection.
Brain cryosections from control and JEV infected animals were stained with antibodies against CD40, CD80, CD86, and MHC class I molecules and Nestin. Isotype staining using rat IgG2aκ (for costimulatory molecules and MHC class I) and normal mouse serum (NMS; for Nestin) was performed on both control (A) and JEV infected brain sections (B). Co-localization of Nestin (red) with the costimulatory molecules and MHC class I (green) was performed using confocal microscopy. Isotype staining on both control and JEV infected sections show no detectable fluorescence (A–B). The Nestin +ve cells show a distinctive morphological change following infection from process bearing cells to round/oval shaped cells. Nestin positive cells in control sections show very less co-localization with all the costimulatory molecules and MHC class I (C, E, G, I). JEV infected SVZ show complete and increased co-localisation of Nestin positive cells with CD40 (D), CD80 (F), CD86 (H), and MHC class I (J). Scale bar corresponds to 20 microns.
Figure 2. Increased surface expression of costimulatory molecules and MHC class I by NSPCs following JEV infection in vitro.
NSPCs isolated from the SVZ were cultured as free-floating neurospheres and infected with JEV at MOI = 5. Following infection, the cells were cultured and collected at different time points from 1 dpi to 4 dpi. Control and JEV infected NSPCs were stained with anti-CD40, anti-CD80, anti-CD86, and anti-MHC class I and isotype controls (IgG2aκ) antibodies, and analyzed using BD FACS Calibur system. The histograms were plotted and the mean fluorescent intensity (MFI) was calculated over all the existing peaks in each of different conditions and finally represented graphically as the averaged MFI for each condition (2A). Significant increase in surface expression of CD40 (2A i, v), CD80 (2A ii, vi) and CD86 (2A iii, viii) was noted at 3 dpi in JEV infected NSPCs. MHC class I (2A iv, viii) expression was upregulated significantly at both 2 and 3 dpi in the infected NSPCs compared to control NSPCs and JEV infected isotype. # indicates p<0.05 compared to control NSPCs and infected isotypes. Control and JEV infected neurospheres at 3 dpi were collected and allowed to adhere on PDL-coated chamber slides for 3 hours and immunostained for CD40, CD80, CD 86 and MHC class I molecules, as well as rat IgG2aκ isotype (2B). The nuclear counterstain was done with DAPI (blue). Increased surface expression of all the costimulatory molecules (CD40, 80, 86) was observed in infected neurospheres. Robust surface staining for MHC class I was noticed in the infected NSPCs compared to uninfected control. Scale bar corresponds to 20 microns.
Figure 3. Production of inflammatory cytokines by NSPCs upon JEV infection.
Control and JEV infected neurospheres were collected at 1–4 dpi and cell lysates were prepared. CBA was performed with the cell lysates and the graphs represent the concentration of the different cytokines produced. Significant production of TNF-α (A), IFN-γ (B), IL-6 (C), and CCL2/MCP-1 (D) was observed in JEV infected NSPCs compared to control NSPCs, and the levels of all cyto/chemokines reached the maximum at 3 dpi and 4 dpi. Values represent the means ± SEM from three independent experiments. * indicates p<0.005 compared to control.
Figure 4. Stimulation of T cell proliferation by NSPCs upon JEV infection.
Control and JEV infected NSPCs were dissociated into single cell suspension and treated with mitomycin C. Purified splenic T cells were used as responders and co-cultured with control and infected NSPCs (stimulators) for 72 hours. MTS reagent was used to detect proliferation and absorbance was measured at 490 nm. Experiments were performed in triplicate. The graph represents the stimulator index (SI), which shows that JEV infected NSPCs have a higher stimulator index, i.e. stimulate greater T cell proliferation compared to control NSPCs (A). * indicates p<0.05 compared to control. Purified splenic T cells were labeled with CFSE and then cultured alone or co-cultured with control and JEV infected NSPCs for 72–80 hours. The cells were then stained for anti-CD3ε antibody and analyzed by FACS. The dot plots represent the population of CD3 detected on FL2 channel and CFSE label on FL1 channel. The decrease of CFSE staining is an indication of proliferation and we quantified the percentage of CD3 +ve cells, which have low CFSE label (upper left quadrant of dot plots) (B). JEV infected NSPCs induced T cell proliferation by 2 fold compared to control NSPCs (p<0.01).
Figure 5. Induction of IL-2 mRNA expression by NSPCs upon JEV infection.
RNA was isolated from control and JEV infected NSPCs at different dpi and cDNA was prepared from them. qRT-PCR was performed for IL-2 mRNA and normalised to 18S rRNA internal control. The graph represents the relative expression of IL-2 in JEV infected NSPCs at different dpi compared to control NSPCs. A significant increase in IL-2 mRNA levels was observed in JEV infected NSPCs from 1 dpi to 3 dpi compared to control NSPCs. * indicates p<0.01 compared to control.
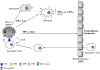
Figure 6. Schematic representation of the possible role of NSPCs in induction of an immune response to virus infection.
Following JEV infection, NSPCs secrete an array of cyto/chemokines which help in the generation of an innate immune response against the viral pathogen. IFN-γ and IL-6 released form JEV infected NSPCs serve to activate the astrocytes and microglia, the primary immune cells of the CNS. Furthermore, both TNF-α and CCL2 have proven roles in upregulation of cell adhesion molecules on the endothelial cells of the blood brain barrier. These cell adhesion molecules help in recruitment of the activated T cells and monocytes from the periphery, which can now easily enter the CNS. On the other hand, JEV infection leads to upregulation of surface expression of costimulatory molecules like CD40, CD80, CD86 as well as MHC class I antigens on NSPCs. These costimulatory molecules and MHC class I on NSPCs are capable of providing functional co-stimulation to T cells and promote their proliferation. Furthermore, infected NSPCs also produce IL-2 which provides mitogenic signals for T cell proliferation. Thus, infected NSPCs are capable of stimulating the adaptive immune system, but whether the formation of an immunological synapse with T cells occurs is still under investigation.
Similar articles
- Japanese encephalitis virus infects neural progenitor cells and decreases their proliferation.
Das S, Basu A. Das S, et al. J Neurochem. 2008 Aug;106(4):1624-36. doi: 10.1111/j.1471-4159.2008.05511.x. Epub 2008 Jun 7. J Neurochem. 2008. PMID: 18540995 - Japanese encephalitis virus infection alters both neuronal and astrocytic differentiation of neural stem/progenitor cells.
Ariff IM, Thounaojam MC, Das S, Basu A. Ariff IM, et al. J Neuroimmune Pharmacol. 2013 Jun;8(3):664-76. doi: 10.1007/s11481-013-9455-7. Epub 2013 Apr 2. J Neuroimmune Pharmacol. 2013. PMID: 23546886 - Regulation of Onecut2 by miR-9-5p in Japanese encephalitis virus infected neural stem/progenitor cells.
Sharma S, Majumdar A, Basu A. Sharma S, et al. Microbiol Spectr. 2024 Mar 5;12(3):e0323823. doi: 10.1128/spectrum.03238-23. Epub 2024 Feb 6. Microbiol Spectr. 2024. PMID: 38319106 Free PMC article. - The involvement of microglial cells in Japanese encephalitis infections.
Thongtan T, Thepparit C, Smith DR. Thongtan T, et al. Clin Dev Immunol. 2012;2012:890586. doi: 10.1155/2012/890586. Epub 2012 Aug 7. Clin Dev Immunol. 2012. PMID: 22919405 Free PMC article. Review. - Immunomodulatory and Anti-inflammatory effect of Neural Stem/Progenitor Cells in the Central Nervous System.
Ni W, Ramalingam M, Li Y, Park JH, Dashnyam K, Lee JH, Bloise N, Fassina L, Visai L, De Angelis MGC, Pedraz JL, Kim HW, Hu J. Ni W, et al. Stem Cell Rev Rep. 2023 May;19(4):866-885. doi: 10.1007/s12015-022-10501-1. Epub 2023 Jan 17. Stem Cell Rev Rep. 2023. PMID: 36650367 Review.
Cited by
- Coxsackievirus preferentially replicates and induces cytopathic effects in undifferentiated neural progenitor cells.
Tsueng G, Tabor-Godwin JM, Gopal A, Ruller CM, Deline S, An N, Frausto RF, Milner R, Crocker SJ, Whitton JL, Feuer R. Tsueng G, et al. J Virol. 2011 Jun;85(12):5718-32. doi: 10.1128/JVI.02261-10. Epub 2011 Apr 6. J Virol. 2011. PMID: 21471247 Free PMC article. - Neurons under viral attack: victims or warriors?
Chakraborty S, Nazmi A, Dutta K, Basu A. Chakraborty S, et al. Neurochem Int. 2010 May-Jun;56(6-7):727-35. doi: 10.1016/j.neuint.2010.02.016. Epub 2010 Mar 4. Neurochem Int. 2010. PMID: 20206655 Free PMC article. Review. - PD1+CCR2+CD8+ T Cells Infiltrate the Central Nervous System during Acute Japanese Encephalitis Virus Infection.
Zhang F, Qi L, Li T, Li X, Yang D, Cao S, Ye J, Wei B. Zhang F, et al. Virol Sin. 2019 Oct;34(5):538-548. doi: 10.1007/s12250-019-00134-z. Epub 2019 Jun 18. Virol Sin. 2019. PMID: 31215000 Free PMC article. - Sodium Butyrate Induced Neural Stem/Progenitor Cell Death in an Experimental Model of Japanese Encephalitis.
Satheesan A, Sharma S, Basu A. Satheesan A, et al. Metab Brain Dis. 2023 Dec;38(8):2831-2847. doi: 10.1007/s11011-023-01279-3. Epub 2023 Aug 31. Metab Brain Dis. 2023. PMID: 37650987 - Abrogated inflammatory response promotes neurogenesis in a murine model of Japanese encephalitis.
Das S, Dutta K, Kumawat KL, Ghoshal A, Adhya D, Basu A. Das S, et al. PLoS One. 2011 Mar 3;6(3):e17225. doi: 10.1371/journal.pone.0017225. PLoS One. 2011. PMID: 21390230 Free PMC article.
References
- Romanko MJ, Rola R, Fike JR, Szele FG, Dizon ML, et al. Roles of the mammalian subventricular zone in cell replacement after brain injury. Prog Neurobiol. 2004;74:77–99. - PubMed
- Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol. 2002;20:1111–1117. - PubMed
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. - PubMed
- Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20:1103–1110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials