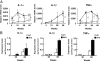Gangliosides play pivotal roles in the regulation of complement systems and in the maintenance of integrity in nerve tissues - PubMed (original) (raw)
Gangliosides play pivotal roles in the regulation of complement systems and in the maintenance of integrity in nerve tissues
Yuhsuke Ohmi et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Gangliosides are considered to be essential in the maintenance and repair of nervous tissues; however, the mechanisms for neurodegeneration caused by ganglioside defects are unknown. We examined gene expression profiles in double knockout (DKO) mice of GM2/GD2 synthase and GD3 synthase genes and showed that the majority of complement genes and their receptors were up-regulated in cerebellum in DKO mice. Inflammatory reactions were demonstrated in those tissues by measuring up-regulated inflammatory cytokines, indicating the presence of complement activation and inflammation as reported in Alzheimer's disease. Immunoblotting of fractionated membrane extracts by sucrose density gradient revealed that complement-regulatory molecules such as decay-accelerating factor and CD59 were dispersed from glycolipid-enriched microdomain/rafts in DKO cerebellum. Immunohistostaining of these molecules showed disordered membrane localization. These results suggested that dysfunction of complement-regulatory molecules may be due to abnormal glycolipid-enriched microdomain/rafts that triggered complement activation, subsequent inflammation, and neurodegeneration in DKO mice. Generation of the triple KO mice lacking complement activity in addition to the two glycosyltransferases suggested that complement activation is involved in the inflammatory reactions and neurodegeneration caused by the ganglioside deficiency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
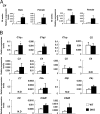
Synthetic pathway of gangliosides and phenotypes of DKO mice. (A) A synthetic pathway of gangliosides. Ganglioside species that are absent in the KO mice for GM2/GD2 synthase and GD3 synthase genes are indicated. Consequently, structures boxed in two squares were depleted in the DKO mice. (B) TLC of gangliosides from cerebella of 15-week-old WT and DKO mice. Resorcinol was used for the detection. (C) Wet weights of the cerebella of 30- and 50- to 65-week-old WT and DKO mice. Cerebella were cleaved at upper edge of inferior cerebellar peduncle after PBS perfusion. Numbers of tissues examined were: 30-week-old WT n = 15, DKO n = 14; 50- to 65-week-old WT n = 13, DKO n = 11; data are presented as mean ± SD. (D) Cerebellum sections of 50-week-old mice stained with HE. Arrowheads indicate Purkinje cells. (E) The numbers of Purkinje cells were counted and presented as the number per 9,000-μm range of the Purkinje layer. The numbers of mice examined were: 8-, 30-, and 50-week-old WT n = 2, DKO n = 2; data are presented as mean ± SD. [Scale bar, 50 μm (D).] *, P < 0.05; ***, P < 0.001.
Fig. 2.
mRNA levels of complement components were up-regulated in the cerebellum of DKO mice. mRNA levels of complement genes in the cerebellum of individual mice were analyzed. (A) Expression of the C4 gene. (B) mRNA levels of 15 complement-related genes in the cerebellum of 28-week-old male mice were analyzed by real time RT-PCR and presented after correction with GAPDH gene. The number of mice examined was: 28-week-old male WT n = 3, DKO n = 3; 28-week-old female WT n = 3, DKO n = 6; 48-week-old male WT n = 3, DKO n = 3; 48-week-old female WT n = 3, DKO n = 5; data are presented as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001. N.D., not detectable.
Fig. 3.
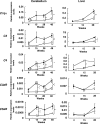
Complement genes were up-regulated with aging in the cerebellum of DKO mice. mRNAs from the cerebella of 4-, 15-, 28-, and 48-week-old mice and from livers of 4-, 15-, and 28-week-old mice were analyzed for the expression levels of five complement genes with real time RT-PCR and presented after correction by the GAPDH gene. Left column, expression levels of _C1q_α, C3, C4, C3aR, and C5aR in the cerebellum. Right column, expression levels in the liver. Open diamond, WT; closed square, DKO. The number of mice examined was: WT n = 3, DKO n = 3; data are presented as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Fig. 4.
Inflammatory cytokines increased in the cerebellum of DKO mice with aging. (A) mRNAs from the cerebellum of individual mice were analyzed for the expression levels of cytokine genes with real time RT-PCR, and corrected by the mouse GAPDH gene. Open diamond, WT; closed square, DKO. The number of mice examined was: 4-, 15-, 30-, and 60-week-old WT n = 3, DKO n = 3. (B) The protein levels of IL-1α, IL-1β, and TNFα in the cerebellum of 30- and 60-week-old mice as analyzed with ELISA. The number of mice examined was: 30- and 60-week-old WT n = 3, DKO n = 2. *, P < 0.05; **, P < 0.01; ***, P < 0.001. N.D., not detectable.
Fig. 5.
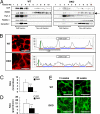
Altered distribution of GPI-anchored complement-regulatory proteins in the cerebellum of DKO mice. (A) Floatation patterns of DAF, N-CAM (a GPI-anchored isoform is indicated by an arrow), Thy1.2, flotillin-1, and caveolin-1 in the cerebellum from 46-week-old mice were analyzed by immunoblotting using fractions separated by sucrose density gradient centrifugation. GEM/rafts were located in fractions 2–4. (B–D) Confocal microscopic image of DAF (B) in the granular cells of the 60-week-old mouse cerebellum. Cellular distribution of DAF was analyzed by scanning images, and the ratios of fluorescence intensities between membrane and cytoplasm (brown/blue bar of B) were calculated (C). The sharpness of the peaks of fluorescence (green/brown bar of B) was also calculated (D). The number of neurons examined was: WT n = 20, DKO n = 20 (D and E); data are presented as mean ± SD. (E) Confocal microscopic images of flotillin-1 in the granular cells of 15- and 60-week-old cerebellum. [Scale bar,10 μm (B and E).] ***, P < 0.001.
Fig. 6.
Alleviation of the complement activation, inflammation, and neurodegeneration in the DKO mice by disruption of complement C3 gene. (A) Expression levels of complement mRNAs from the cerebella of 15-week-old mice were analyzed for _C1q_α and C3 genes with real time RT-PCR. Relative expression levels are presented after correction by the GAPDH gene. The numbers of mice examined were: 15-week-old WT n = 5, DKO n = 4, TKO n = 3, C3 KO n = 3. (B) Deposits of C1q in the cerebella of 15-week-old WT, DKO, TKO, and C3 KO mice were analyzed with anti-C1q mAb in combination with Alexa Flour 488-conjugated anti-rat IgG. Fluorescence intensity (mean pixel × total area) of complement staining was measured by digital image analysis. Number of section area examined was: 15-week-old WT n = 6, DKO n = 6 TKO n = 6, C3 KO n = 6; data are presented as mean ± SD. The thickness of sections was 7 μm. (Scale bar, 20 μm.) (C) Expression levels of cytokine mRNAs (_IL-1_α, _IL-1_β, and _TNF_α) were analyzed with real time RT-PCR using RNAs from the cerebellum of individual mice. Relative expression levels are presented after correction by the mouse GAPDH gene. The number of mice examined was: 15-week-old WT n = 4, DKO n = 4, TKO n = 3, C3 KO n = 3. [Scale bar, 50 μm (A and B).] Thickness of sections, 7 μm. (D) Wet weights of the cerebella of 15-week-old WT, DKO and TKO mice as measured in Fig. 1. Numbers of tissues examined were: WT n = 6, DKO n = 5, TKO n = 3, C3 KO n = 3; data are presented as mean ± SD. (E) Cerebellar sections of 30-week-old mice stained with HE. Arrowheads indicate Purkinje cells. The numbers of Purkinje cells were counted and presented as in Fig. 1. The numbers of mice examined were: 30-week-old WT n = 2, DKO n = 2 TKO n = 2, C3 KO n = 2. Data are presented as mean ± SD. (Scale bar, 50 μm.) *, P < 0.05; ***, P < 0.001.
Similar articles
- Gangliosides are essential in the protection of inflammation and neurodegeneration via maintenance of lipid rafts: elucidation by a series of ganglioside-deficient mutant mice.
Ohmi Y, Tajima O, Ohkawa Y, Yamauchi Y, Sugiura Y, Furukawa K, Furukawa K. Ohmi Y, et al. J Neurochem. 2011 Mar;116(5):926-35. doi: 10.1111/j.1471-4159.2010.07067.x. Epub 2011 Jan 12. J Neurochem. 2011. PMID: 21214571 - Ganglioside deficiency causes inflammation and neurodegeneration via the activation of complement system in the spinal cord.
Ohmi Y, Ohkawa Y, Tajima O, Sugiura Y, Furukawa K, Furukawa K. Ohmi Y, et al. J Neuroinflammation. 2014 Mar 28;11:61. doi: 10.1186/1742-2094-11-61. J Neuroinflammation. 2014. PMID: 24673754 Free PMC article. - Regulatory mechanisms of nervous systems with glycosphingolipids.
Furukawa K, Ohmi Y, Ohkawa Y, Tokuda N, Kondo Y, Tajima O, Furukawa K. Furukawa K, et al. Neurochem Res. 2011 Sep;36(9):1578-86. doi: 10.1007/s11064-011-0494-2. Epub 2011 May 12. Neurochem Res. 2011. PMID: 21562747 Review. - Essential roles of gangliosides in the formation and maintenance of membrane microdomains in brain tissues.
Ohmi Y, Ohkawa Y, Yamauchi Y, Tajima O, Furukawa K, Furukawa K. Ohmi Y, et al. Neurochem Res. 2012 Jun;37(6):1185-91. doi: 10.1007/s11064-012-0764-7. Epub 2012 Apr 10. Neurochem Res. 2012. PMID: 22488331 Review. - Glycolipids: Essential regulator of neuro-inflammation, metabolism and gliomagenesis.
Furukawa K, Ohmi Y, Ji S, Zhang P, Bhuiyan RH, Ohkawa Y, Tajima O, Hashimoto N, Furukawa K. Furukawa K, et al. Biochim Biophys Acta Gen Subj. 2017 Oct;1861(10):2479-2484. doi: 10.1016/j.bbagen.2017.06.007. Epub 2017 Jun 7. Biochim Biophys Acta Gen Subj. 2017. PMID: 28602513 Review.
Cited by
- Strong antibody reaction against glycosphingolipids injected in liposome-embedded forms in beta3GN-T5 knockout mice.
Fan X, Kondo Y, Tokuda N, Ohmi Y, Ando R, Umezu T, Zhang Q, Furukawa K, Shibata K, Togayachi A, Narimatsu H, Okajima T, Kikkawa K, Furukawa K. Fan X, et al. Nagoya J Med Sci. 2011 Aug;73(3-4):137-46. Nagoya J Med Sci. 2011. PMID: 21928695 Free PMC article. - Structures, biosynthesis, and functions of gangliosides--an overview.
Yu RK, Tsai YT, Ariga T, Yanagisawa M. Yu RK, et al. J Oleo Sci. 2011;60(10):537-44. doi: 10.5650/jos.60.537. J Oleo Sci. 2011. PMID: 21937853 Free PMC article. Review. - Stem cell glycolipids.
Yanagisawa M. Yanagisawa M. Neurochem Res. 2011 Sep;36(9):1623-35. doi: 10.1007/s11064-010-0358-1. Epub 2010 Dec 16. Neurochem Res. 2011. PMID: 21161592 Review. - The Regulatory Roles of Cerebellar Glycosphingolipid Microdomains/Lipid Rafts.
Komatsuya K, Kikuchi N, Hirabayashi T, Kasahara K. Komatsuya K, et al. Int J Mol Sci. 2023 Mar 14;24(6):5566. doi: 10.3390/ijms24065566. Int J Mol Sci. 2023. PMID: 36982638 Free PMC article. Review. - Profiling of patient-specific myocytes identifies altered gene expression in the ophthalmoplegic subphenotype of myasthenia gravis.
Nel M, Prince S, Heckmann JM. Nel M, et al. Orphanet J Rare Dis. 2019 Jan 29;14(1):24. doi: 10.1186/s13023-019-1003-y. Orphanet J Rare Dis. 2019. PMID: 30696470 Free PMC article.
References
- Wiegandt H. Gangliosides. In: Wiegandt H, editor. Glycolipids. New York: Elsevier; 1985. pp. 199–260.
- Furukawa K, Tajima O, Okuda T, Tokuda N, Furukawa K. Knockout mice and glycolipids. In: Kamerling JP, et al., editors. Comprehensive Glycoscience. From Chemistry to Systems Biology. Oxford, UK: Elsevier; 2007. pp. 149–157.
- Kittaka D, et al. Impaired hypoglossal nerve regeneration in complex ganglioside-lacking mutant mice: Down-regulation of neurotrophic factors and receptors as possible mechanisms. Glycobiology. 2008;18:509–516. - PubMed
- Sugiura Y, et al. Sensory nerve-dominant nerve degeneration and remodeling in the mutant mice lacking complex gangliosides. Neuroscience. 2005;135:1167–1178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous