p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis - PubMed (original) (raw)
. 2010 Jan 29;327(5965):593-6.
doi: 10.1126/science.1166202. Epub 2009 Dec 17.
Philip M Santiago, Emmanuelle di Tomaso, Julie M Sullivan, Wu-Shiun Hou, Talya Dayton, Laura B Jeffords, Pooja Sodha, Kim L Mercer, Rhianna Cohen, Osamu Takeuchi, Stanley J Korsmeyer, Roderick T Bronson, Carla F Kim, Kevin M Haigis, Rakesh K Jain, Tyler Jacks
Affiliations
- PMID: 20019247
- PMCID: PMC2897160
- DOI: 10.1126/science.1166202
p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis
David G Kirsch et al. Science. 2010.
Erratum in
- Science. 2011 Nov 11;334(6057):761
Abstract
Acute exposure to ionizing radiation can cause lethal damage to the gastrointestinal (GI) tract, a condition called the GI syndrome. Whether the target cells affected by radiation to cause the GI syndrome are derived from the epithelium or endothelium and whether the target cells die by apoptosis or other mechanisms are controversial issues. Studying mouse models, we found that selective deletion of the proapoptotic genes Bak1 and Bax from the GI epithelium or from endothelial cells did not protect mice from developing the GI syndrome after sub-total-body gamma irradiation. In contrast, selective deletion of p53 from the GI epithelium, but not from endothelial cells, sensitized irradiated mice to the GI syndrome. Transgenic mice overexpressing p53 in all tissues were protected from the GI syndrome after irradiation. These results suggest that the GI syndrome is caused by the death of GI epithelial cells and that these epithelial cells die by a mechanism that is regulated by p53 but independent of apoptosis.
Figures
Fig. 1
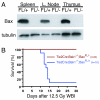
Deletion of the intrinsic pathway of apoptosis in the hematopoietic system protects mice from death after whole-body gamma irradiation (WBI). A. Western blot for BAX and tubulin in spleen, lymph node, and thymus from Tie2Cre; Bak1−/−; BaxFL/− mice (FL/−) demonstrates deletion of Bax in the hematopoietic system. Tie2Cre; Bak1−/−; BaxFL/+ mice (FL/+) retain Bax and serve as a control. B. Kaplan-Meier survival analysis of Tie2Cre; Bak1−/−; BaxFL/+ mice and Tie2Cre; Bak1−/−; BaxFL/− litter mates after 12.5 Gy WBI. By log-rank comparison, p = 0.0001.
Fig. 2
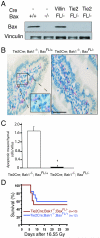
Deletion of the intrinsic pathway of apoptosis from endothelial cells does not protect mice from subtotal-body gamma irradiation (SBI). A. Western blot for BAX and Vinculin from lung endothelial cells isolated from mice that contained no Cre (−), VillinCre (Villin), or Tie2Cre (Tie2) and the indicated Bax genotype. B. Mesenchymal cell (endothelial cell and lymphocyte) apoptosis is decreased in Tie2Cre; Bak1−/−; BaxFL/− mice. TUNEL (brown) and MECA-32 (pink) immunohistochemistry identify apoptotic mesenchymal cells within the villi of Tie2Cre; Bak1−/−; BaxFL/+ mice. Scale bar, 100 μm. C. Quantification of mesenchymal cell apoptosis in the villi of the distal small intestine. The mean number of mesenchymal cells undergoing apoptosis in a total of at least 87 villi from 3 mice is shown; *p < 0.01 by two-tailed t test. Error bars indicate SEM. D. Kaplan-Meier survival analysis of Tie2Cre; Bak1−/−; BaxFL/+ mice and Tie2Cre; Bak1−/−; BaxFL/− litter mates after 16.55 Gy SBI. By log-rank comparison, p =NS.
Fig. 3
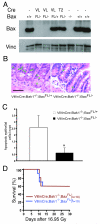
Deletion of the intrinsic pathway of apoptosis from GI epithelial cells does not protect mice from subtotal-body gamma irradiation (SBI). A. Western blot for BAX and Vinculin from GI epithelial cells isolated from mice without Cre (−), with VillinCre (VL), or Tie2Cre (T2), and the indicated Bax genotype. B. Hematoxylin and eosin stained sections of distal small intestine 4 hours after 16.95 Gy SBI demonstrates suppression of apoptosis within the crypt epithelium in VillinCre; Bak1−/−; BaxFL/− mice. A green asterisk labels cells with characteristic apoptotic morphology. Scale bar, 100 μm. C. Quantification of GI epithelial cell apoptosis in the crypts of the distal small intestine. The mean number of apoptotic cells in a total of at least 100 crypts in 3 mice is shown. Error bars indicate SEM. *p < 0.01, by two-tailed t test. Similar results were observed in the proximal small intestine. D. Kaplan-Meier survival analysis of VillinCre; Bak1−/−; BaxFL/+ and VillinCre; Bak1−/−; BaxFL/− mice after 16.95 Gy SBI. By log-rank comparison, p = NS.
Fig. 4
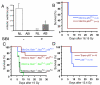
Regulation of the GI syndrome by p53 in epithelial cells is independent of apoptosis. A. Quantification of normal (NL) and aberrant (AB) mitoses in GI crypt epithelial cells of the proximal and distal small intestine from wild-type mice without (−) or 24 hours after (+) 13.4 Gy SBI with ionizing radiation. The mean number of mitoses per crypt in a total of at least 115 crypts from 6 mice is shown. Error bars indicate SEM. *p < 0.04 for aberrant mitoses, two-sided t test and **p < 0.005 for normal mitoses, two-sided t test. B. Kaplan-Meier survival analysis of VillinCre; p53FL/+ and VillinCre; p53FL/− mice after 16.15 Gy SBI with gamma irradiation. p = 0.0015 by log-rank test. C. Kaplan-Meier survival analysis of at least 9 mice of each of the indicated genotype after 15 Gy SBI with gamma irradiation. p = 0.0001 between p53FL/− and p53FL/+ mice and p = NS between BaxFL/− and BaxFL/+ mice by log-rank test. D. Kaplan-Meier survival analysis of “super p53” tgb mice and C57/BL6 p53+/+ littermate controls after 13.4 Gy SBI with ionizing radiation. p = 0.02 by log-rank test.
Similar articles
- Bax and Bak do not exhibit functional redundancy in mediating radiation-induced endothelial apoptosis in the intestinal mucosa.
Rotolo JA, Maj JG, Feldman R, Ren D, Haimovitz-Friedman A, Cordon-Cardo C, Cheng EH, Kolesnick R, Fuks Z. Rotolo JA, et al. Int J Radiat Oncol Biol Phys. 2008 Mar 1;70(3):804-15. doi: 10.1016/j.ijrobp.2007.11.043. Epub 2008 Jan 11. Int J Radiat Oncol Biol Phys. 2008. PMID: 18191336 - Radiation-induced gastric epithelial apoptosis occurs in the proliferative zone and is regulated by p53, bak, bax, and bcl-2.
Przemeck SM, Duckworth CA, Pritchard DM. Przemeck SM, et al. Am J Physiol Gastrointest Liver Physiol. 2007 Feb;292(2):G620-7. doi: 10.1152/ajpgi.00391.2006. Epub 2006 Oct 26. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17068116 - Acidic polysaccharide of Panax ginseng as a defense against small intestinal damage by whole-body gamma irradiation of mice.
Park E, Hwang I, Song JY, Jee Y. Park E, et al. Acta Histochem. 2011 Jan;113(1):19-23. doi: 10.1016/j.acthis.2009.07.003. Epub 2009 Sep 19. Acta Histochem. 2011. PMID: 19767060 - Morphological aspects of ionizing radiation response of small intestine.
Somosy Z, Horváth G, Telbisz A, Réz G, Pálfia Z. Somosy Z, et al. Micron. 2002;33(2):167-78. doi: 10.1016/s0968-4328(01)00013-0. Micron. 2002. PMID: 11567886 Review. - [Problems of radiobiology and p53 protein].
Mazurik VK, Moroz BB. Mazurik VK, et al. Radiats Biol Radioecol. 2001 Sep-Oct;41(5):548-72. Radiats Biol Radioecol. 2001. PMID: 11721349 Review. Russian.
Cited by
- Single-molecule transcript counting of stem-cell markers in the mouse intestine.
Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van Es J, Lees J, Jacks T, Clevers H, van Oudenaarden A. Itzkovitz S, et al. Nat Cell Biol. 2011 Nov 27;14(1):106-14. doi: 10.1038/ncb2384. Nat Cell Biol. 2011. PMID: 22119784 Free PMC article. - Pro-apoptotic gene knockdown mediated by nanocomplexed siRNA reduces radiation damage in primary salivary gland cultures.
Arany S, Xu Q, Hernady E, Benoit DS, Dewhurst S, Ovitt CE. Arany S, et al. J Cell Biochem. 2012 Jun;113(6):1955-65. doi: 10.1002/jcb.24064. J Cell Biochem. 2012. PMID: 22253051 Free PMC article. - Low- and high-LET radiation drives clonal expansion of lung progenitor cells in vivo.
Farin AM, Manzo ND, Kirsch DG, Stripp BR. Farin AM, et al. Radiat Res. 2015 Jan;183(1):124-32. doi: 10.1667/RR13878.1. Epub 2015 Jan 7. Radiat Res. 2015. PMID: 25564721 Free PMC article. - An inducible long noncoding RNA amplifies DNA damage signaling.
Schmitt AM, Garcia JT, Hung T, Flynn RA, Shen Y, Qu K, Payumo AY, Peres-da-Silva A, Broz DK, Baum R, Guo S, Chen JK, Attardi LD, Chang HY. Schmitt AM, et al. Nat Genet. 2016 Nov;48(11):1370-1376. doi: 10.1038/ng.3673. Epub 2016 Sep 26. Nat Genet. 2016. PMID: 27668660 Free PMC article. - The p53 pathway in hematopoiesis: lessons from mouse models, implications for humans.
Pant V, Quintás-Cardama A, Lozano G. Pant V, et al. Blood. 2012 Dec 20;120(26):5118-27. doi: 10.1182/blood-2012-05-356014. Epub 2012 Sep 27. Blood. 2012. PMID: 23018641 Free PMC article. Review.
References
- Mettler FA, Voelz GL. N. Engl. J. Med. 2002;346:1554. - PubMed
- van Bekkum DW, Schotman E. Int. J. Radiat. Biol. 1974;25:361. - PubMed
- Terry NHA, Travis EL. Int. J. Radiat. Onc. Biol. Phys. 1989;17:569. - PubMed
- Mason KA, Withers HR, McBride WH, Davis CA, Smathers JB. Radiation Res. 1989;117:480. - PubMed
- Brown JM. Int. J. Radiat. Onc. Bio. Phys. 2008;70:799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 CA114176/CA/NCI NIH HHS/United States
- RC1-AI078521/AI/NIAID NIH HHS/United States
- RC1 AI078521-01/AI/NIAID NIH HHS/United States
- U19-AI06775/AI/NIAID NIH HHS/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- P30-CA14051/CA/NCI NIH HHS/United States
- P01 CA080124/CA/NCI NIH HHS/United States
- P01 CA80124/CA/NCI NIH HHS/United States
- P01 CA080124-01A1/CA/NCI NIH HHS/United States
- P30 CA014051-38/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- K08 CA 114176/CA/NCI NIH HHS/United States
- K08 CA114176-05/CA/NCI NIH HHS/United States
- RC1 AI078521/AI/NIAID NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous