The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5 - PubMed (original) (raw)
. 2010 Jan;120(1):127-41.
doi: 10.1172/JCI40027. Epub 2009 Dec 14.
Twan van den Beucken, Ludwig Dubois, Hanneke Niessen, Johan Bussink, Kim Savelkouls, Tom Keulers, Hilda Mujcic, Willy Landuyt, Jan Willem Voncken, Philippe Lambin, Albert J van der Kogel, Marianne Koritzinsky, Bradly G Wouters
Affiliations
- PMID: 20038797
- PMCID: PMC2798689
- DOI: 10.1172/JCI40027
The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5
Kasper M A Rouschop et al. J Clin Invest. 2010 Jan.
Abstract
Tumor hypoxia is a common microenvironmental factor that adversely influences tumor phenotype and treatment response. Cellular adaptation to hypoxia occurs through multiple mechanisms, including activation of the unfolded protein response (UPR). Recent reports have indicated that hypoxia activates a lysosomal degradation pathway known as autophagy, and here we show that the UPR enhances the capacity of hypoxic tumor cells to carry out autophagy, and that this promotes their survival. In several human cancer cell lines, hypoxia increased transcription of the essential autophagy genes microtubule-associated protein 1 light chain 3beta (MAP1LC3B) and autophagy-related gene 5 (ATG5) through the transcription factors ATF4 and CHOP, respectively, which are regulated by PKR-like ER kinase (PERK, also known as EIF2AK3). MAP1LC3B and ATG5 are not required for initiation of autophagy but mediate phagophore expansion and autophagosome formation. We observed that transcriptional induction of MAP1LC3B replenished MAP1LC3B protein that was turned over during extensive hypoxia-induced autophagy. Correspondingly, cells deficient in PERK signaling failed to transcriptionally induce MAP1LC3B and became rapidly depleted of MAP1LC3B protein during hypoxia. Consistent with these data, autophagy and MAP1LC3B induction occurred preferentially in hypoxic regions of human tumor xenografts. Furthermore, pharmacological inhibition of autophagy sensitized human tumor cells to hypoxia, reduced the fraction of viable hypoxic tumor cells, and sensitized xenografted human tumors to irradiation. Our data therefore demonstrate that the UPR is an important mediator of the hypoxic tumor microenvironment and that it contributes to resistance to treatment through its ability to facilitate autophagy.
Figures
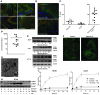
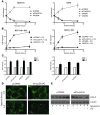
Figure 1. Autophagy is induced by hypoxia and is primarily present in hypoxic tumor regions.
(A) Immunohistochemical staining of 12 head and neck tumor cell lines (SCCNij) for MAP1LC3B (red), hypoxia (pimonidazole [pimo], green), and vessels (9F1, blue) revealed that MAP1LC3B staining was primarily localized to hypoxic regions. Percentage colocalization of MAP1LC3B and pimonidazole is indicated in the bottom right corner. Representative micrographs are shown at low magnification (×10). (B) Higher magnification (×400) revealed a punctate MAP1LC3B pattern characteristic of autophagosome formation. (C) Quantification of the 12 head and neck tumor cell lines for hypoxia, MAP1LC3B staining, and MAP1LC3B expressed specifically in hypoxic regions showed that MAP1LC3B in tumors is primarily expressed in hypoxic regions of tumors. (D) Enrichment factor (enrichment compared with the statistical probability) on log scale for 12 head and neck tumor cell lines. (E) Immunoblots for MAP1LC3B on MCF7, U373, and HCT116 lysates showed processing and increased expression of MAP1LC3B during hypoxic (<0.02% O2) exposure. (F) Immunohistochemical staining for MAP1LC3B on U373 cells exposed to 1 hour of hypoxia showed redistribution of MAP1LC3B and an increased punctate pattern indicative of induction of autophagy. Original magnification, ×800. (G) Confirmation of autophagy induction during hypoxia by electron microscopy. Numerous autophagosomes were detected after anoxic exposure (8 hours) of U373 cells (original magnification, ×6,300). High magnification (inset, ×21,500) clearly revealed the double membrane, characteristic of autophagosomes. N, nucleus. (H) Immunoblots on HT29 lysates showed rapid activation of MAP1LC3B during hypoxia. CQ addition identified a very high autophagic flux during hypoxia. (I) Flow cytometric assessment of MAP1LC3B levels during hypoxia (filled circles), hypoxia in the presence of CQ (open circles), or normoxic exposure in the presence of CQ (open squares) identified a very high autophagic flux during hypoxia (data are presented as mean ± SEM, n = 3).
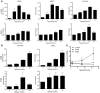
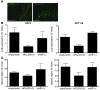
Figure 2. Regulation of autophagy genes MAP1LC3B and ATG5 during hypoxic exposure.
(A) Validation of the obtained microarray results by quantitative real-time PCR confirmed MAP1LC3B and ATG5 upregulation during hypoxia (<0.02% O2). n = 3, mean ± SEM. (B) Confirmation of MAP1LC3B and ATG5 gene regulation by quantitative real-time PCR during hypoxia in HCT116 and U373 cells. n = 3, mean ± SEM. (C) mRNA abundance in polysomal fractions indicated translation of MAP1LC3B and ATG5 during hypoxia (<0.02% O2). n = 2, mean ± SD.
Figure 3. Induction of MAP1LC3B and ATG5 mRNA is dependent on PERK signaling.
(A and B) Knockdown of HIF-1α, PERK, IRE-1, or ATF6 in HCT116 and U373 cells showed that hypoxia-dependent induction (<0.02% O2 for 24 hours) of MAP1LC3B (A) and ATG5 (B) is dependent on PERK signaling. Samples were normalized to normoxic exposure (control) and compared with scrambled (SCR) siRNA knockdown. n = 3 mean ± SEM. (C) MAP1LC3B and ATG5 dependency on UPR signaling was further confirmed in HCT116 and U373 cells hampered in UPR signaling. n = 3, mean ± SEM. (D) Knockdown of ATF4 prevented upregulation of MAP1LC3B mRNA during hypoxia. Control was normoxic exposure. n = 3, mean ± SEM. (E) Knockdown of ATF4 and its downstream transcription factor CHOP prevented ATG5 mRNA upregulation during hypoxia. n = 3, mean ± SEM. (F) CHOP knockdown reduced ATG5 protein expression during normoxia and hypoxia as detected by immunoblotting.
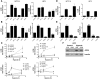
Figure 4. The UPR transcription factors ATF4 and CHOP bind the MAP1LC3B and the ATG5 promoters, respectively.
(A) Locations of primer pairs used for quantitative real-time PCR analysis following ChIP. (B) ATF4 ChIP analysis revealed enrichment of putative ATF4 transcription factor binding sites in MAP1LC3B (A primer) but not at a region further upstream (a primer) in both HCT116 and U373 cell lines. Enrichment of the CHOP promoter was used as a positive control for ATF4. (C) CHOP ChIP enriched the promoters of ATG5 (B primer) but not at a region further upstream (b primer) in both lines. Enrichment of GADD34 was used as a positive control for CHOP. HA denotes the signal obtained after HA-ChIP followed by PCR for the respective MAP1LC3B promoter or the ATG5 promoter. t = 0, 1, or 4 indicates the amount of time (in hours) exposed to hypoxia (<0.02% O2).
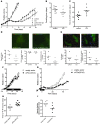
Figure 5. UPR signaling is required for maintenance of autophagy during hypoxia.
(A) Inhibition of UPR signaling caused depletion of MAP1LC3B protein during hypoxic exposure as measured by flow cytometry. (B) Addition of CQ led to the accumulation of MAP1LC3B and prevented depletion of MAP1LC3B in UPR-deficient cells during hypoxia. (C) Addition of CQ to cells exposed to normoxia led to a 1.5- to 2-fold accumulation of MAP1LC3B. All data are n = 3, mean ± SEM. (D) Immunohistochemical staining for MAP1LC3B of HCT116-pCDNA5 and –pCDNA5-eIF2α(S51A) cells under normal conditions or after 4 hours of hypoxia (<0.02% O2). Original magnification, ×600. (E) Immunoblots for MAP1LC3B on HCT116-pCDNA5 and –pCDNA5-eIF2α(S51A) lysates during hypoxia (<0.02% O2). CQ was added as a control for autophagic flux.
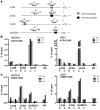
Figure 6. UPR signaling is required for MAP1LC3B expression in hypoxic areas of tumor xenografts.
(A) Representative images of control (pCDNA3) of UPR-inhibited pCDNA3-eIF2α(S51A) xenografts. MAP1LC3B is displayed in red, hypoxia (pimonidazole) in green, and vessels (9F1) in blue. Original magnification, ×10. (B) Quantification of MAP1LC3B expression and hypoxia showed decreased expression of MAP1LC3B in pCDNA3-eIF2α(S51A) xenografts. Knockdown of HIF-1α had no effect on MAP1LC3B expression in hypoxic regions. n = 4, mean ± SEM. (C) Expression of pCDNA3-eIF2α(S51A) decreased the hypoxic regions in xenografts. Knocking down HIF-1α had no effect. n = 4, mean ± SEM.
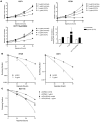
Figure 7. Inhibition of autophagy by CQ addition sensitizes cells to hypoxia.
(A) Growth curves under normoxic and moderately hypoxic (0.2% O2) conditions showed that CQ addition selectively inhibited cell proliferation in U373, HT29, and HCT116 cells under hypoxic conditions (0.2% O2). Lower right: Quantification of the CQ-mediated inhibition of cell proliferation during normoxic and hypoxic conditions. n = 3, mean ± SEM. (B and C) Clonogenic survival after hypoxic (<0.02% O2) exposure with or without the addition of CQ in HT29 and U373 cells (B) or HCT116-pCDNA3 and HCT116–pCDNA3-eIF2α(S51A) cells (C). n = 3, mean ± SEM.
Figure 8. CQ treatment of tumors decreases the hypoxic fraction and sensitizes to radiation.
(A) Growth curves of HCT116 xenografts: untreated (filled circles; saline, n = 10), treated from day –4 to +3 with CQ (60 mg/kg) (open circles; CQ, n = 7), irradiated at day 0 with a single, tumor-specific dose of 10 Gy (filled squares; saline, 10 Gy, n = 7), and treated from day –4 to +3 with CQ and irradiated with 10 Gy (open squares; CQ, 10 Gy, n = 8). (B) Doubling times of unirradiated tumors treated or untreated with CQ. (C) Time to reach 4-fold irradiated tumor volume. (D) Top: Micrographs of LC3/pimonidazole/vessel staining after 7 days of CQ or saline treatment. Original magnification, ×10. Bottom: Hypoxic fraction, MAP1LC3B/pimonidazole colocalization, and total MAP1LC3B staining in the sections (n = 5). (E) Top: Proliferation (BrdU) and pimonidazole staining of tumor sections after 7 days of CQ or saline treatment (n = 5). Bottom: Quantification of overall proliferation and proliferation in hypoxic regions. (F) HCT116 isogenic xenografts harboring doxycycline-inducible genes [empty vector or eIF2α(S51A)] were established in nude mice. When tumors reached a size of 150 mm3, mice were fed doxycycline (2 g/l) continuously and tumor growth was assessed [empty vector, n = 6; eIF2α(S51A), n = 5; mean ± SEM]. (G) HCT116 isogenic xenografts [empty vector, n = 7; eIF2α(S51A), n = 8] were established in nude mice and irradiated with 10 Gy when they reached 150 mm3 (day 0). Doxycycline (2 g/l) was administered for 7 days (day –4 to +3) to induce the transgene, and tumor size was assessed as a function of time (mean ± SEM). (H) Time for tumors in G to reach 4 times the irradiated volume. (I) The hypoxic fraction in individual isogenic xenografts was determined after 7 days of doxycycline treatment using pimonidazole immunohistochemistry.
Figure 9. Model for recycling and regeneration of MAP1LC3B during hypoxia.
During the process of autophagy, the extending membrane (phagophore) requires coating with MAP1LC3B to form an autophagosome. The ATG5-ATG12-ATG16 complex redistributes and recruits MAP1LC3B to the membrane. Before degradation of the autolysosomal content, the ATG5-ATG12-ATG16 complex and the MAP1LC3B at the outer membrane is released to be recycled and the portion of MAP1LC3B inside the autolysosome is degraded. Hypoxia activates autophagy through BNIP3/BNIP3L signaling or AMPK (initiation). Activation of UPR/PERK signaling increases the capacity to maintain autophagy as it replenishes the overturned MAP1LC3B, but this activation also increases ATG5 expression to form ATG5-ATG12-ATG16 complexes.
Similar articles
- Hepatitis C virus core protein activates autophagy through EIF2AK3 and ATF6 UPR pathway-mediated MAP1LC3B and ATG12 expression.
Wang J, Kang R, Huang H, Xi X, Wang B, Wang J, Zhao Z. Wang J, et al. Autophagy. 2014 May;10(5):766-84. doi: 10.4161/auto.27954. Epub 2014 Feb 20. Autophagy. 2014. PMID: 24589849 Free PMC article. - Cell death induced by the ER stressor thapsigargin involves death receptor 5, a non-autophagic function of MAP1LC3B, and distinct contributions from unfolded protein response components.
Lindner P, Christensen SB, Nissen P, Møller JV, Engedal N. Lindner P, et al. Cell Commun Signal. 2020 Jan 27;18(1):12. doi: 10.1186/s12964-019-0499-z. Cell Commun Signal. 2020. PMID: 31987044 Free PMC article. - Regulation of autophagy by ATF4 in response to severe hypoxia.
Rzymski T, Milani M, Pike L, Buffa F, Mellor HR, Winchester L, Pires I, Hammond E, Ragoussis I, Harris AL. Rzymski T, et al. Oncogene. 2010 Aug 5;29(31):4424-35. doi: 10.1038/onc.2010.191. Epub 2010 May 31. Oncogene. 2010. PMID: 20514020 - Role of ATF4 in regulation of autophagy and resistance to drugs and hypoxia.
Rzymski T, Milani M, Singleton DC, Harris AL. Rzymski T, et al. Cell Cycle. 2009 Dec;8(23):3838-47. doi: 10.4161/cc.8.23.10086. Epub 2009 Dec 15. Cell Cycle. 2009. PMID: 19887912 Review. - The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth.
Fels DR, Koumenis C. Fels DR, et al. Cancer Biol Ther. 2006 Jul;5(7):723-8. doi: 10.4161/cbt.5.7.2967. Epub 2006 Jul 1. Cancer Biol Ther. 2006. PMID: 16861899 Review.
Cited by
- Protective or Harmful: The Dual Roles of Autophagy in Diabetic Retinopathy.
Gong Q, Wang H, Yu P, Qian T, Xu X. Gong Q, et al. Front Med (Lausanne). 2021 Mar 25;8:644121. doi: 10.3389/fmed.2021.644121. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33842506 Free PMC article. Review. - Blockage of Autophagy for Cancer Therapy: A Comprehensive Review.
Hassan AMIA, Zhao Y, Chen X, He C. Hassan AMIA, et al. Int J Mol Sci. 2024 Jul 7;25(13):7459. doi: 10.3390/ijms25137459. Int J Mol Sci. 2024. PMID: 39000565 Free PMC article. Review. - The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies.
Luo B, Lee AS. Luo B, et al. Oncogene. 2013 Feb 14;32(7):805-18. doi: 10.1038/onc.2012.130. Epub 2012 Apr 16. Oncogene. 2013. PMID: 22508478 Free PMC article. Review. - Regulation of cellular immunity by activating transcription factor 4.
Mukherjee D, Bercz LS, Torok MA, Mace TA. Mukherjee D, et al. Immunol Lett. 2020 Dec;228:24-34. doi: 10.1016/j.imlet.2020.09.006. Epub 2020 Sep 28. Immunol Lett. 2020. PMID: 33002512 Free PMC article. Review. - Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy.
Poillet-Perez L, Despouy G, Delage-Mourroux R, Boyer-Guittaut M. Poillet-Perez L, et al. Redox Biol. 2015;4:184-92. doi: 10.1016/j.redox.2014.12.003. Epub 2014 Dec 10. Redox Biol. 2015. PMID: 25590798 Free PMC article. Review.
References
- Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38(2):285–289. - PubMed
- Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56(19):4509–4515. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials