BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination - PubMed (original) (raw)
. 2010 Jan;120(1):157-67.
doi: 10.1172/JCI40070. Epub 2009 Dec 28.
Affiliations
- PMID: 20038811
- PMCID: PMC2799219
- DOI: 10.1172/JCI40070
BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination
Laurent Derré et al. J Clin Invest. 2010 Jan.
Abstract
The function of antigen-specific CD8+ T cells, which may protect against both infectious and malignant diseases, can be impaired by ligation of their inhibitory receptors, which include CTL-associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1). Recently, B and T lymphocyte attenuator (BTLA) was identified as a novel inhibitory receptor with structural and functional similarities to CTLA-4 and PD-1. BTLA triggering leads to decreased antimicrobial and autoimmune T cell responses in mice, but its functions in humans are largely unknown. Here we have demonstrated that as human viral antigen-specific CD8+ T cells differentiated from naive to effector cells, their surface expression of BTLA was gradually downregulated. In marked contrast, human melanoma tumor antigen-specific effector CD8+ T cells persistently expressed high levels of BTLA in vivo and remained susceptible to functional inhibition by its ligand herpes virus entry mediator (HVEM). Such persistence of BTLA expression was also found in tumor antigen-specific CD8+ T cells from melanoma patients with spontaneous antitumor immune responses and after conventional peptide vaccination. Remarkably, addition of CpG oligodeoxynucleotides to the vaccine formulation led to progressive downregulation of BTLA in vivo and consequent resistance to BTLA-HVEM-mediated inhibition. Thus, BTLA activation inhibits the function of human CD8+ cancer-specific T cells, and appropriate immunotherapy may partially overcome this inhibition.
Figures
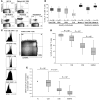
Figure 1. BTLA (CD272) is downregulated with progressive CD8+ T cell differentiation, except in tumor antigen–specific CD8+ T cells.
Direct ex vivo analysis of CD8+ T cells from PBMCs by flow cytometry. (A) Representative examples of BTLA expression by Melan-AMART-1– and virus-specific (EBV, CMV, and influenza virus [FLU]) CD8+ T cells from healthy donors (HD) and melanoma patients. BTLA+ T cells were distinguished from BTLA– T cells using a threshold established according to the autologous naive T cell subset, which is always BTLA positive. Numbers indicate percentage of positive or negative cells. (B) Comparison of BTLA expression on total CD8+ and virus- and Melan-A–specific T cells, from healthy individuals and unvaccinated melanoma patients. **P < 10–2 for each of the 2 populations of Melan-A–specific T cells compared with all 5 other populations. (C–E) BTLA expression in subsets of CD8+ T cells of healthy individuals (n = 58), upon definition of early and late differentiation stages by CD45RA and CCR7 expression (i.e. naive [N, CD45RA+CCR7+], central memory [CM, CD45RA–CCR7+], effector memory [EM, CD45RA–CCR7–], and effector memory RA+ [EMRA, CD45RA+CCR7–] cells), gated as shown in C; a representative example demonstrates the narrow positive peak observed in phenotypic naive cells. (D) Statistical assessment of BTLA+ CD8+ T cell subsets. (E) GMFI data, showing results compatible with those presented inD. Overall GMFI was determined by including the whole range from BTLA-negative to -positive cells, as shown in C. GMFI data were normalized to values determined in autologous naive CD8+ T cells.
Figure 2. Persistence of BTLA expression on Melan-AMART-1–specific lymphocytes despite effector cell differentiation.
(A) Analysis of BTLA expression by Melan-AMART-1–specific T cells depending on differentiation. Representative example of PBMCs from an unvaccinated melanoma patient. (B) Percentages of BTLA+ CD8+ T cells from healthy individuals (gray, n = 58) and unvaccinated melanoma patients (white, n = 18); and from Melan-AMART-1 tetramer+ CD8+ T cells from unvaccinated patients (black,n = 14). Data from healthy donors (same as in Figure 1D) are shown again for comparison. Circulating central memory Melan-AMART-1–specific cells were rare, precluding analysis for BTLA expression. (C and D) BTLA expression by CD45RA+/– and CCR7+/– T cells from normal lymph nodes (NLN, n = 18) and tumor-infiltrated lymph nodes (TILN, n = 6) from melanoma patients. (C) Representative example and (D) comparison of BTLA expression in CD8+ T cells and Melan-AMART-1–specific T cells. In NLNs, Melan-AMART-1–specific T cells are all naive. Light gray, whole CD8+ T cells in NLNs (n = 18); white, Melan-AMART-1 tetramer+ CD8+ T cells in NLNs (n = 18); black, whole CD8+ T cells in TILNs (n = 6); dark gray, Melan-AMART-1 tetramer+ CD8+ T cells in TILNs (n = 6).
Figure 3. Expression of HVEM and its ligands BTLA and LIGHT by melanoma cells; and BTLA-mediated functional inhibition of tumor antigen–specific BTLA+ CD8+ T cells.
(A) Representative examples of BTLA, LIGHT, and HVEM expression by the melanoma cell lines Me 280, Me 261, and Me 290. Cells were stained with isotype control (open histograms) or with anti-HVEM, anti-BTLA, or anti-LIGHT mAb (filled histograms) (B) Relative expression of HVEM, BTLA, and LIGHT by melanoma cell lines, expressed as ratio fluorescence intensity (RFI), i.e., MFI with specific mAb/MFI with isotype control. Of 40 melanoma cell lines analyzed, 19 were highly positive (white dots), 7 weakly positive (gray dots), and 14 negative for HVEM expression (black dots). None of the melanoma cell lines expressed BTLA or LIGHT (black dots). (C) HVEM expression (in reddish brown) detected in paraffin-embedded tumor sections from 16 tumors of 14 melanoma patients. Examples show tumor tissues that were HVEM-negative, weakly positive (<10% HVEM+ tumor cells) or strongly positive (>50% positive tumor cells). Original magnification, ×200. (D) Melan-AMART-1/HLA-A*0201–specific CD8+ T cell clones (cl.) 618-45, 618-4, and 618-420 were stimulated by melanoma cell lines Me 275 and Me 290 (both Melan-A+, HLA-A*0201+, HVEM+). The graph shows fold increase in IFN-γ production by Melan-AMART-1–specific clones in the presence of blocking antibody BTLA-8.2, relative to isotype control antibody. IFN-γ production was determined by ELISA in supernatants of 24-hour cultures. BTLA expression by T cell clones was assessed by flow cytometry with BTLA-specific antibody (filled histograms) and isotype control (open histograms). GMFI is indicated in parentheses.
Figure 4. Repeated vaccination with Melan-AMART-1 peptide, IFA (Montanide ISA-51), and CpG (PF-3512676) induces downregulation of BTLA on Melan-A–specific CD8+ T cells.
(A) Representative examples of A2/Melan-AMART-1 tetramer and BTLA staining in unvaccinated and vaccinated patients. Numbers indicate percentages of positive or negative cells. w/o, without. (B) Comparison of BTLA expression in Melan-AMART-1–specific CD8+ T cells from 14 unvaccinated patients, 24 patients vaccinated with peptide but without CpG, and 16 patients vaccinated with peptide plus CpG. (C) Expression of BTLA by Melan-AMART-1–specific CD8+ T cells (black) and whole CD8+ T cells (white) according to CD8 differentiation stages after vaccinations without CpG (upper panel; n = 24) or with CpG (lower panel; n = 16).
Figure 5. Progressive BTLA downregulation upon repeated vaccination with CpG.
(A) Representative dot plots of A2/Melan-AMART-1 tetramer and BTLA staining (gated on CD8+ T cells) of PBMCs from 2 patients, before vaccination and after 16 vaccinations, respectively. Numbers indicate percentages of positive or negative cells. (B and C) Correlations between numbers of vaccinations without or with CpG and percentages of BTLA+ T cells among (B) Melan-A–specific CD8+ T cells and whole CD8+ T cells and (C) subsets of Melan-A–specific CD8+ T cells.
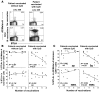
Figure 6. Functional inhibition of CD8+ T cells depending on BTLA expression and HVEM triggering.
(A) Direct ex vivo analysis of percentage of A2/Melan-AMART-1 tetramer+ CD8+ T cells among PBMCs, in relation to their BTLA expression, after vaccination without and with CpG. (B) In vitro expansion of Melan-AMART-1 tetramer+ T cells from healthy donors, after 10 days of stimulation with peptide-loaded DCs, in the absence versus presence of blocking mAb BTLA-8.2. (C and D) IFN-γ (C) and TNF-α (D) production by BTLA+ antigen-specific CD8+ T cells after 4 hours of peptide stimulation of PBMCs assessed ex vivo, i.e., without prior in vitro cultivation. Representative histograms of PBMCs from patient LAU 618 (after 2 vaccinations, with CpG) and from healthy donor BCB122 stimulated by SKMEL37 cells expressing HVEM or not, loaded with Melan-AMART-1 or EBV peptides, respectively. Histograms are gated on tetramer+ T cells. Percentages of cytokine-positive cells are indicated. (E) Significant correlation between percentages of BTLA+ tetramer+ T cells and percentages of IFN-γ production inhibition (i.e., reduction when stimulated with HVEM+ SKMEL37 cells as compared with HVEM– SKMEL37 cells). Each dot represents a single experiment with Melan-AMART-1– or EBV-specific T cells.
Figure 7. Downregulation of BTLA by primary CD8+ T cells upon T cell receptor activation and HVEM ligation.
(A) Representative example of BTLA downregulation by BTLA+ EBV-specific CD8+ T cells from PBMCs of healthy donor BCB136 upon EBV peptide stimulation with target cells expressing HVEM or not. Histograms are gated on CD8+ EBV+ T cells. Percentages of BTLA+ cells (and GMFI in parentheses) are indicated. (B) Percentages of BTLA expression by antigen-specific CD8+ T cells after stimulation with SKMEL37 cells expressing HVEM or not. Each dot represents a single experiment with Melan-AMART-1– or EBV-specific T cells. (C and D) Kinetic analysis of surface BTLA expression (C) and expansion (D) of EBV-specific CD8+ T cells upon stimulation with irradiated target cells expressing HVEM or not, in the presence or absence of EBV peptide. Results for 2 of 5 representative healthy donors tested are shown.
Comment in
- Putting the brakes on BTLA in T cell-mediated cancer immunotherapy.
Paulos CM, June CH. Paulos CM, et al. J Clin Invest. 2010 Jan;120(1):76-80. doi: 10.1172/JCI41811. Epub 2009 Dec 28. J Clin Invest. 2010. PMID: 20038807 Free PMC article.
Similar articles
- B and T lymphocyte attenuator mediates inhibition of tumor-reactive CD8+ T cells in patients after allogeneic stem cell transplantation.
Hobo W, Norde WJ, Schaap N, Fredrix H, Maas F, Schellens K, Falkenburg JH, Korman AJ, Olive D, van der Voort R, Dolstra H. Hobo W, et al. J Immunol. 2012 Jul 1;189(1):39-49. doi: 10.4049/jimmunol.1102807. Epub 2012 May 25. J Immunol. 2012. PMID: 22634623 - Putting the brakes on BTLA in T cell-mediated cancer immunotherapy.
Paulos CM, June CH. Paulos CM, et al. J Clin Invest. 2010 Jan;120(1):76-80. doi: 10.1172/JCI41811. Epub 2009 Dec 28. J Clin Invest. 2010. PMID: 20038807 Free PMC article. - CpG-ODN-induced sustained expression of BTLA mediating selective inhibition of human B cells.
Thibult ML, Rivals JP, Mamessier E, Gertner-Dardenne J, Pastor S, Speiser DE, Derré L, Olive D. Thibult ML, et al. J Mol Med (Berl). 2013 Feb;91(2):195-205. doi: 10.1007/s00109-012-0943-7. Epub 2012 Aug 19. J Mol Med (Berl). 2013. PMID: 22903545 Clinical Trial. - BTLA and HVEM cross talk regulates inhibition and costimulation.
Gavrieli M, Sedy J, Nelson CA, Murphy KM. Gavrieli M, et al. Adv Immunol. 2006;92:157-85. doi: 10.1016/S0065-2776(06)92004-5. Adv Immunol. 2006. PMID: 17145304 Review. - The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation.
Cai G, Freeman GJ. Cai G, et al. Immunol Rev. 2009 May;229(1):244-58. doi: 10.1111/j.1600-065X.2009.00783.x. Immunol Rev. 2009. PMID: 19426226 Review.
Cited by
- The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer.
Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. Muenst S, et al. Breast Cancer Res Treat. 2013 Jun;139(3):667-76. doi: 10.1007/s10549-013-2581-3. Epub 2013 Jun 12. Breast Cancer Res Treat. 2013. PMID: 23756627 Free PMC article. - B and T lymphocyte attenuator down-regulation by HIV-1 depends on type I interferon and contributes to T-cell hyperactivation.
Zhang Z, Xu X, Lu J, Zhang S, Gu L, Fu J, Jin L, Li H, Zhao M, Zhang J, Wu H, Su L, Fu YX, Wang FS. Zhang Z, et al. J Infect Dis. 2011 Jun 1;203(11):1668-78. doi: 10.1093/infdis/jir165. J Infect Dis. 2011. PMID: 21592997 Free PMC article. - PD-1 Blockade Promotes Emerging Checkpoint Inhibitors in Enhancing T Cell Responses to Allogeneic Dendritic Cells.
Stecher C, Battin C, Leitner J, Zettl M, Grabmeier-Pfistershammer K, Höller C, Zlabinger GJ, Steinberger P. Stecher C, et al. Front Immunol. 2017 May 22;8:572. doi: 10.3389/fimmu.2017.00572. eCollection 2017. Front Immunol. 2017. PMID: 28588576 Free PMC article. - Expression profiling of TCR-engineered T cells demonstrates overexpression of multiple inhibitory receptors in persisting lymphocytes.
Abate-Daga D, Hanada K, Davis JL, Yang JC, Rosenberg SA, Morgan RA. Abate-Daga D, et al. Blood. 2013 Aug 22;122(8):1399-410. doi: 10.1182/blood-2013-04-495531. Epub 2013 Jul 16. Blood. 2013. PMID: 23861247 Free PMC article. - The nature of activatory and tolerogenic dendritic cell-derived signal II.
Bakdash G, Sittig SP, van Dijk T, Figdor CG, de Vries IJ. Bakdash G, et al. Front Immunol. 2013 Feb 28;4:53. doi: 10.3389/fimmu.2013.00053. eCollection 2013. Front Immunol. 2013. PMID: 23450201 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials