Clinical and immunological responses in metastatic melanoma patients vaccinated with a high-dose poly-epitope vaccine - PubMed (original) (raw)
Clinical Trial
doi: 10.1007/s00262-009-0811-7. Epub 2009 Dec 31.
Paul Lorigan, Ulrich Keilholz, Dirk Schadendorf, Adrian Harris, Christian Ottensmeier, John Smyth, Klaus Hoffmann, Richard Anderson, Martin Cripps, Joerg Schneider, Robert Hawkins
Affiliations
- PMID: 20043222
- PMCID: PMC11030722
- DOI: 10.1007/s00262-009-0811-7
Clinical Trial
Clinical and immunological responses in metastatic melanoma patients vaccinated with a high-dose poly-epitope vaccine
Adam Dangoor et al. Cancer Immunol Immunother. 2010 Jun.
Abstract
Background: Safety and cellular immunogenicity of rising doses and varying regimens of a poly-epitope vaccine were evaluated in advanced metastatic melanoma. The vaccine comprised plasmid DNA and recombinant modified vaccinia virus Ankara (MVA) both expressing a string (Mel3) of seven HLA.A2/A1 epitopes from five melanoma antigens.
Methods: Forty-one HLA-A2 positive patients with stage III/IV melanoma were enrolled. Patient groups received one or two doses of DNA.Mel3 followed by escalating doses of MVA.Mel3. Immunisations then continued eight weekly in the absence of disease progression. Epitope-specific CD8+ T cell responses were evaluated using ex-vivo tetramer and IFN-gamma ELISPOT assays. Safety and clinical responses were monitored.
Results: Prime-boost DNA/MVA induced Melan-A-specific CD8+ T cell responses in 22/31 (71%) patients detected by tetramer assay. ELISPOT detected a response to at least one epitope in 10/31 (32%) patients. T cell responder rates were <50% with low-dose DNA/MVA, or MVA alone, rising to 91% with high-dose DNA/MVA. Among eight patients showing evidence of clinical benefit-one PR (24 months+), five SD (5 months+) and two mixed responses-seven had associated immune responses. Melan-A-tetramer+ immunity was associated with a median 8-week increase in time-to-progression (P = 0.037) and 71 week increase in survival (P = 0.0002) compared to non-immunity. High-dose vaccine was well tolerated. The only significant toxicities were flu-like symptoms and injection-site reactions.
Conclusions: DNA.Mel3 and MVA.Mel3 in a prime-boost protocol generated high rates of immune response to melanoma antigen epitopes. The treatment was well tolerated and the correlation of immune responses with patient outcomes encourages further investigation.
Figures
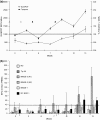
Fig. 1
Melan-A tetramer assay profiles for individual patients treated with higher dose regimens. a Group 5: two 2 mg DNA.Mel3 + two 1 × 109 pfu MVA.Mel3, b Group 7: one 4 mg DNA.Mel3 + two 1 × 109 pfu MVA.Mel3, and c Group 6: four 1 × 109 pfu MVA.Mel3. Immunisations at 3-week intervals. Dotted line signifies mean + 2 standard deviations (SD) of baseline response (above line positive response). open triangles administration of DNA.Mel3, closed triangles MVA.Mel3
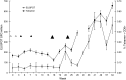
Fig. 2
T cell immune responses elicited against multiple melanoma epitopes in Patient #47. a Mean Melan-A specific response detected by tetramer assay (circles) and ELISPOT assay (squares) ± 1 SD, b Response against epitopes other than Melan-A detected by ELISPOT assay. Dotted line mean +2 SD of baseline response in the tetramer assay, dashed line mean +3 SD of the negative control responses in the ELISPOT assay, open triangles priming immunisations with 2 mg pSG2.Mel3, closed triangles boosting immunisations with 5 × 108 pfu MVA.Mel3
Fig. 3
Enhanced Melan-A specific T cell responses elicited in Patient #33 after extended boosting with MVA.Mel3. Mean Melan-A specific response detected by tetramer assay (circles) and ELISPOT assay (squares) ±1 SD. T cell immune responses were not detected against other epitopes (data not shown). Initial prime with two 2 mg pSG.Mel3 (open triangles) and boost with two 1 × 107 pfu MVA.Mel3 (solid triangles). Two additional boosters of a higher dose 2 × 108 pfu MVA.Mel3 on weeks 18 and 25 (large solid triangles)
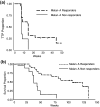
Fig. 4
Kaplan-Meier survival curves showing Melan-A tetramer responders compared to non-responders. a Per protocol analysis of time-to-progression (TTP) for stage III and IV patients (29 subjects) receiving prime-boost regime (Groups 1–5 and 7), b Intention to treat analysis of survival for stage III and IV patients (31 subjects) receiving prime-boost regime (Groups 1–5 and 7). Open circles censored patients
Similar articles
- Recombinant modified vaccinia Ankara primes functionally activated CTL specific for a melanoma tumor antigen epitope in melanoma patients with a high risk of disease recurrence.
Smith CL, Dunbar PR, Mirza F, Palmowski MJ, Shepherd D, Gilbert SC, Coulie P, Schneider J, Hoffman E, Hawkins R, Harris AL, Cerundolo V. Smith CL, et al. Int J Cancer. 2005 Jan 10;113(2):259-66. doi: 10.1002/ijc.20569. Int J Cancer. 2005. PMID: 15386406 Clinical Trial. - Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma.
Weber J, Boswell W, Smith J, Hersh E, Snively J, Diaz M, Miles S, Liu X, Obrocea M, Qiu Z, Bot A. Weber J, et al. J Immunother. 2008 Feb-Mar;31(2):215-23. doi: 10.1097/CJI.0b013e3181611420. J Immunother. 2008. PMID: 18481391 Clinical Trial. - Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes.
Goonetilleke N, Moore S, Dally L, Winstone N, Cebere I, Mahmoud A, Pinheiro S, Gillespie G, Brown D, Loach V, Roberts J, Guimaraes-Walker A, Hayes P, Loughran K, Smith C, De Bont J, Verlinde C, Vooijs D, Schmidt C, Boaz M, Gilmour J, Fast P, Dorrell L, Hanke T, McMichael AJ. Goonetilleke N, et al. J Virol. 2006 May;80(10):4717-28. doi: 10.1128/JVI.80.10.4717-4728.2006. J Virol. 2006. PMID: 16641265 Free PMC article. Clinical Trial. - The use of HLA class I tetramers to design a vaccination strategy for melanoma patients.
Palmowski M, Salio M, Dunbar RP, Cerundolo V. Palmowski M, et al. Immunol Rev. 2002 Oct;188:155-63. doi: 10.1034/j.1600-065x.2002.18814.x. Immunol Rev. 2002. PMID: 12445289 Review. - The paradox of T-cell-mediated antitumor immunity in spite of poor clinical outcome in human melanoma.
Anichini A, Vegetti C, Mortarini R. Anichini A, et al. Cancer Immunol Immunother. 2004 Oct;53(10):855-64. doi: 10.1007/s00262-004-0526-8. Epub 2004 Jun 3. Cancer Immunol Immunother. 2004. PMID: 15175905 Free PMC article. Review.
Cited by
- Constant regulation for stable CD8 T-cell functional avidity and its possible implications for cancer immunotherapy.
Gilfillan CB, Hebeisen M, Rufer N, Speiser DE. Gilfillan CB, et al. Eur J Immunol. 2021 Jun;51(6):1348-1360. doi: 10.1002/eji.202049016. Epub 2021 Mar 30. Eur J Immunol. 2021. PMID: 33704770 Free PMC article. Review. - Phase I trial of recombinant modified vaccinia ankara encoding Epstein-Barr viral tumor antigens in nasopharyngeal carcinoma patients.
Hui EP, Taylor GS, Jia H, Ma BB, Chan SL, Ho R, Wong WL, Wilson S, Johnson BF, Edwards C, Stocken DD, Rickinson AB, Steven NM, Chan AT. Hui EP, et al. Cancer Res. 2013 Mar 15;73(6):1676-88. doi: 10.1158/0008-5472.CAN-12-2448. Epub 2013 Jan 24. Cancer Res. 2013. PMID: 23348421 Free PMC article. Clinical Trial. - Elevated tumor-associated antigen expression suppresses variant peptide vaccine responses.
Kemmler CB, Clambey ET, Kedl RM, Slansky JE. Kemmler CB, et al. J Immunol. 2011 Nov 1;187(9):4431-9. doi: 10.4049/jimmunol.1101555. Epub 2011 Sep 21. J Immunol. 2011. PMID: 21940675 Free PMC article. - Therapeutic cancer vaccines in prostate cancer: the quest for intermediate markers of response.
Kim JW, Bilusic M, Heery CJ, Madan RA. Kim JW, et al. Cancers (Basel). 2012 Nov 22;4(4):1229-46. doi: 10.3390/cancers4041229. Cancers (Basel). 2012. PMID: 24213505 Free PMC article. - Programmed cell death-1 (PD-1) at the heart of heterologous prime-boost vaccines and regulation of CD8+ T cell immunity.
Bot A, Qiu Z, Wong R, Obrocea M, Smith KA. Bot A, et al. J Transl Med. 2010 Dec 14;8:132. doi: 10.1186/1479-5876-8-132. J Transl Med. 2010. PMID: 21144062 Free PMC article.
References
- King M, Spooner D, Rowlands DC. Spontaneous regression of metastatic malignant melanoma of the parotid gland and neck lymph nodes: a case report and a review of the literature. Clin Oncol (R Coll Radiol) 2001;13:466–469. - PubMed
- Itoh K, Platsoucas CD, Balch CM. Autologous tumor-specific cytotoxic T lymphocytes in the infiltrate of human metastatic melanomas: activation by interleukin 2 and autologous tumor cells, and involvement of the T cell receptor. J Exp Med. 1988;168:1419–1441. doi: 10.1084/jem.168.4.1419. - DOI - PMC - PubMed
- Schwartzentruber DJ, Topalian SL, Mancini M, Rosenberg SA. Specific release of granulocyte-macrophage colony-stimulating factor, tumor necrosis factor-alpha, and IFN-gamma by human tumor-infiltrating lymphocytes after autologous tumor stimulation. J Immunol. 1991;146:3674–3681. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials