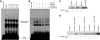15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis requires Src-mediated Egr-1-dependent rapid induction of FGF-2 expression - PubMed (original) (raw)
15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis requires Src-mediated Egr-1-dependent rapid induction of FGF-2 expression
Venkatesh Kundumani-Sridharan et al. Blood. 2010.
Erratum in
Abstract
To understand the mechanisms underlying 15(S)-hydroxyeicosatetraenoic acid [15(S)-HETE]-induced angiogenesis, we studied the role of Egr-1. 15(S)-HETE induced Egr-1 expression in a time-dependent manner in human dermal microvascular endothelial cells (HDMVECs). Blockade of Egr-1 via forced expression of its dominant-negative mutant attenuated 15(S)-HETE-induced HDMVEC migration and tube formation as well as Matrigel plug angiogenesis. 15(S)-HETE-induced Egr-1 expression requires Src activation. In addition, adenovirus-mediated expression of dominant-negative mutant of Src blocked 15(S)-HETE's effects on migration and tube formation of HDMVECs and Matrigel plug angiogenesis. 15(S)-HETE induced fibroblast growth factor-2 (FGF-2) expression rapidly via Src-mediated production of Egr-1. Cloning and mutational analysis of FGF-2 promoter revealed that Egr-1 binding site proximal to transcription start site is required for 15(S)-HETE-induced FGF-2 expression. Neutralizing antibody-mediated suppression of FGF-2 function also attenuated the effects of 15(S)-HETE on HDMVEC migration and tube formation as well as Matrigel plug angiogenesis. Furthermore, in contrast to wild-type mice, 12/15-LOX(-/-) mice exhibited decreased Matrigel plug angiogenesis in response to AA, which was rescued by 15(S)-HETE. On the basis of these observations, we conclude that 15(S)-HETE-induced angiogenesis requires Src-mediated Egr-1-dependent rapid induction of FGF-2. These findings may suggest that 15(S)-HETE could be a potential endogenous regulator of pathologic angiogenesis associated with atherosclerosis and restenosis.
Figures
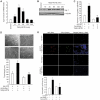
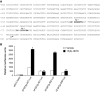
Figure 1
Egr-1 mediates 15(S)-HETE–induced HDMVEC migration and tube-like structure formation in vitro and Matrigel plug angiogenesis in vivo. (A-B) Quiescent human dermal microvascular endothelial cells (HDMVECs) were treated with and without 15(S)-hydroxyeicosatetraenoic acid (15(S)-HETE; 0.1μM) for the indicated time periods, and either RNA was isolated or cell extracts were prepared. (A) RNA was analyzed by QRT-PCR for Egr-1 and β-actin mRNA levels. (B) Cell extracts were analyzed by Western blotting for Egr-1 levels by the use of its specific antibodies. The blot was reprobed with anti–β-tubulin antibodies for normalization. (C-D) HDMVECs were transduced with Ad-GFP or Ad-dnEgr-1 at a moi of 40, quiesced, and subjected to 15(S)-HETE–induced migration (C) or tube-like structure formation (D). (E) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5μM 15(S)-HETE along with and without Ad-GFP or Ad-dnEgr-1 (5 × 109 pfu/mL). One week later, the animals were sacrificed, and the Matrigel plugs were harvested from underneath the skin and either processed for von Willebrand Factor (vWF) and CD31 expression by double immunofluorescence staining with their specific antibodies or analyzed for hemoglobin content with Drabkin reagent. The bar graphs in panels A, C, D, and E represent the quantitative analysis of 3 independent experiments or 6 plugs from 6 animals. The values are presented as the mean ± SD. *P < .01 vs control or Ad-GFP; **P < .01 vs Ad-GFP + 15(S)-HETE.
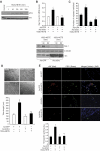
Figure 2
Src mediates 15(S)-HETE–induced Egr-1 expression in HDMVECs, leading to their migration and tube-like structure formation. (A) Quiescent HDMVECs were treated with and without 15(S)-HETE (0.1μM) for the indicated time periods, and cell extracts were prepared and analyzed by Western blotting for pSrc with the use of its phosphospecific antibodies. The blot was reprobed with anti-Src antibodies for normalization. (B) HDMVECs were transduced with Ad-GFP or Ad-dnSrc at a moi of 40, quiesced, and treated with and without 15(S)-HETE (0.1μM) for either 30 minutes for RNA isolation or 30 minutes and 60 minutes for cell extract preparation. RNA was analyzed for Egr-1 and β- actin mRNA levels by QRT-PCR, and cell extracts were analyzed by Western blotting for Egr-1 with the use of its specific antibodies. The blot was reprobed sequentially with anti–β-tubulin antibodies and anti-Src antibodies for normalization and to show the overexpression of dnSrc, respectively. (C-D) HDMVECs that were transduced with Ad-GFP or Ad-dnSrc at a moi of 40 and quiesced were subjected to 15(S)-HETE (0.1μM)–induced migration (C) or tube-like structure formation (D). (E) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5μM 15(S)-HETE in combination with and without Ad-GFP or Ad-dnSrc (5 × 109 pfu/mL). One week later, the animals were sacrificed, and the Matrigel plugs were harvested from underneath the skin and either processed for vWF and CD31 expression by double immunofluorescence staining with their specific antibodies or analyzed for hemoglobin content using Drabkin reagent. The bar graphs in panels B, C, D, and E represent the quantitative analysis of 3 independent experiments or 6 plugs from 6 animals. The values are presented as the mean ± SD. *P < .01 vs Ad-GFP; **P < .01 vs Ad-GFP + 15(S)-HETE.
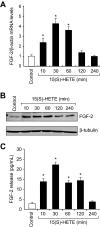
Figure 3
15(S)-HETE induces FGF-2 expression rapidly in HDMVECs. (A-B) Quiescent HDMVECs were treated with and without 15(S)-HETE (0.1μM) for various time periods, and either RNA was isolated or cell extracts were prepared. RNA was analyzed by QRT-PCR for FGF-2 and β-actin mRNA levels, and cell extracts were analyzed by Western blotting for FGF-2 levels with the use of its specific antibodies. The blot was reprobed with anti–β-tubulin antibodies for normalization. (C) All the conditions were the same as in panel B except that medium was collected and analyzed for FGF-2 release by ELISA. The bar graphs in panels A and C represent the quantitative analysis of 3 independent experiments. The values are presented as the means ± SD. *P < .01 vs control.
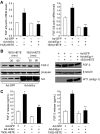
Figure 4
15(S)-HETE–induced FGF-2 expression is mediated by Src-Egr-1 signaling in HDMVECs. (A-B) HDMVECs were transduced with Ad-GFP, Ad-dnSrc, or Ad-dnEgr-1 with a moi of 40, quiesced, and treated with and without 15(S)-HETE (0.1μM) for either 30 minutes for RNA isolation or 30 minutes and/or 60 minutes for cell extract preparation. RNA was analyzed by QRT-PCR for FGF-2 and β-actin mRNA levels, and cell extracts were analyzed by Western blotting for FGF-2 levels with its specific antibodies. The blots were reprobed with anti–β-tubulin antibodies and/or anti-Src antibodies or anti-WT1 antibodies for normalization and to show overexpression of dnSrc or dnEgr-1, respectively. (C) All the conditions were the same as in panel A except that medium was collected and tested for FGF-2 release by ELISA. The bar graphs in panels A and C represent the quantitative analysis of 3 independent experiments. The values are presented as the means ± SD. *P < .01 vs Ad-GFP; **P < .01 vs Ad-GFP + 15(S)-HETE.
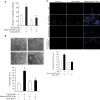
Figure 5
15(S)-HETE–induced FGF-2 promoter-luciferase expression requires Egr-1-binding site. (A) Sequence of a 0.5-kb cloned human FGF-2 promoter region showing the Egr-1 binding sites. Sequence in bold represents Egr-1-binding site; +1 indicates transcription start site. (B) HDMVECs were transfected with either empty vector or FGF-2 promoter-luciferase constructs, quiesced, and treated with and without 0.1μM 15(S)-HETE for 30 minutes, and cell extracts were prepared and analyzed for luciferase activity. The bar graph in panel B represents the quantitative analysis of 3 independent experiments. The values are presented as the means ± SD. *P < .01 vs hFGF2p-WT-Luc vehicle control; **P < .01 vs hFGF2p-WT-Luc + 15(S)-HETE. hFGF2p-WT-Luc indicates human FGF-2 promoter-luciferase construct; hFGF2p-M1-Luc, human FGF-2 promoter-luciferase construct with mutation in the first Egr-1 binding site from the transcriptional start site; hFGF2p-M2-Luc, human FGF-2 promoter-luciferase construct with mutation in the second Egr-1 binding site from the transcriptional start site; hFGF2p-DM-Luc, human FGF-2 promoter-luciferase construct with mutations in both the Egr-1 binding sites.
Figure 6
Egr-1 binds to FGF-2 promoter in response to 15(S)-HETE both in vitro and in vivo in Src-dependent manner. (A,C) Quiescent HDMVECs were treated with and without 15(S)-HETE (0.1μM) for the indicated time periods, and either nuclear extracts were prepared and analyzed by EMSA for DNA binding activity in vitro with the use of [32P]-labeled double stranded oligonucleotide probe that corresponds to the Egr-1 binding site proximal to the transcriptional start site in the FGF-2 promoter (A) or processed for ChIP analysis of Egr-1 binding to FGF-2 promoter in vivo (C). (B,D) HDMVECs that were transduced with Ad-GFP, Ad-dnSrc, or Ad-dnEgr-1 with a moi of 40 and quiesced were treated with and without 15(S)-HETE (0.1μM) for 30 minutes and either nuclear extracts were prepared and analyzed by EMSA for DNA binding activity as described in panel A (B) or processed for ChIP analysis of Egr-1 binding to FGF-2 promoter in vivo (D).
Figure 7
FGF-2 mediates 15(S)-HETE–induced angiogenesis. (A-B) Quiescent HDMVECs were treated with normal serum or neutralizing anti–FGF-2 antibodies (3 μg/mL) for 30 minutes at 37°C followed by washing with medium 131. The cells were then subjected to 15(S)-HETE (0.1μM)–induced migration (A) or tube-like structure formation (B) in the presence and absence of 3 μg/mL of preimmune serum or neutralizing anti–FGF-2 antibodies. (C) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5μM 15(S)-HETE in combination with 20 μg/mL preimmune serum or neutralizing anti–FGF-2 antibodies. One week later, the animals were sacrificed, and the Matrigel plugs were harvested from underneath the skin and either processed for vWF and CD31 expression by double immunofluorescence staining using their specific antibodies or analyzed for hemoglobin content using Drabkin reagent. The bar graphs represent the quantitative analysis of 3 independent experiments or 6 plugs from 6 animals. The values are presented as the mean ± SD. *P < .01 vs control (normal serum); **P < .01 vs 15(S)-HETE (normal serum + 15(S)-HETE).
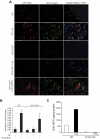
Figure 8
12/15-LOX−/− mice show lack of angiogenic response to AA. (A-B) Wild-type and 12/15-LOX−/− mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle, 5μM AA, or 5μM 15(S)-HETE as indicated. One week later, the animals were sacrificed, and the Matrigel plugs were harvested from underneath the skin and either processed for vWF and CD31 by double immunofluorescence staining by the use of their specific antibodies (A) or analyzed for hemoglobin content with Drabkin reagent (B). (C) Wild-type and 12/15-LOX−/− mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5μM AA. One week later, the Matrigel plugs were retrieved from animals, minced, incubated with 10μM AA and 10μM ionomycin, and 15(S)-HETE production was measured by EIA. The bar graphs represent the quantitative analysis of 6 plugs from 6 animals. The values are presented as the mean ± SD. *P < .01 vs WT control. **P < .01 vs WT control or 12/15-LOX−/− control or 12/15-LOX−/− + AA.
Similar articles
- AP-1 (Fra-1/c-Jun)-mediated induction of expression of matrix metalloproteinase-2 is required for 15S-hydroxyeicosatetraenoic acid-induced angiogenesis.
Singh NK, Quyen DV, Kundumani-Sridharan V, Brooks PC, Rao GN. Singh NK, et al. J Biol Chem. 2010 May 28;285(22):16830-43. doi: 10.1074/jbc.M110.106187. Epub 2010 Mar 29. J Biol Chem. 2010. PMID: 20353950 Free PMC article. - 12/15-Lipoxygenase gene knockout severely impairs ischemia-induced angiogenesis due to lack of Rac1 farnesylation.
Singh NK, Kundumani-Sridharan V, Rao GN. Singh NK, et al. Blood. 2011 Nov 17;118(20):5701-12. doi: 10.1182/blood-2011-04-347468. Epub 2011 Aug 12. Blood. 2011. PMID: 21841162 Free PMC article. - The 15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis requires Janus kinase 2-signal transducer and activator of transcription-5B-dependent expression of interleukin-8.
Cheranov SY, Wang D, Kundumani-Sridharan V, Karpurapu M, Zhang Q, Chava KR, Rao GN. Cheranov SY, et al. Blood. 2009 Jun 4;113(23):6023-33. doi: 10.1182/blood-2008-10-183210. Epub 2009 Apr 6. Blood. 2009. PMID: 19349617 Free PMC article. - A novel role for activating transcription factor-2 in 15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis.
Zhao T, Wang D, Cheranov SY, Karpurapu M, Chava KR, Kundumani-Sridharan V, Johnson DA, Penn JS, Rao GN. Zhao T, et al. J Lipid Res. 2009 Mar;50(3):521-533. doi: 10.1194/jlr.M800388-JLR200. Epub 2008 Oct 10. J Lipid Res. 2009. PMID: 18849464 Free PMC article. - Vascular actions of 20-HETE.
Hoopes SL, Garcia V, Edin ML, Schwartzman ML, Zeldin DC. Hoopes SL, et al. Prostaglandins Other Lipid Mediat. 2015 Jul;120:9-16. doi: 10.1016/j.prostaglandins.2015.03.002. Epub 2015 Mar 23. Prostaglandins Other Lipid Mediat. 2015. PMID: 25813407 Free PMC article. Review.
Cited by
- Inhibiting delta-6 desaturase activity suppresses tumor growth in mice.
He C, Qu X, Wan J, Rong R, Huang L, Cai C, Zhou K, Gu Y, Qian SY, Kang JX. He C, et al. PLoS One. 2012;7(10):e47567. doi: 10.1371/journal.pone.0047567. Epub 2012 Oct 24. PLoS One. 2012. PMID: 23112819 Free PMC article. - Aspirin inhibits the production of proangiogenic 15(S)-HETE by platelet cyclooxygenase-1.
Rauzi F, Kirkby NS, Edin ML, Whiteford J, Zeldin DC, Mitchell JA, Warner TD. Rauzi F, et al. FASEB J. 2016 Dec;30(12):4256-4266. doi: 10.1096/fj.201600530R. Epub 2016 Sep 15. FASEB J. 2016. PMID: 27633788 Free PMC article. - Suppression of transcription factor early growth response 1 reduces herpes simplex virus 1-induced corneal disease in mice.
Yao HW, Chen SH, Li C, Tung YY, Chen SH. Yao HW, et al. J Virol. 2012 Aug;86(16):8559-67. doi: 10.1128/JVI.00505-12. Epub 2012 May 30. J Virol. 2012. PMID: 22647700 Free PMC article. - AP-1 (Fra-1/c-Jun)-mediated induction of expression of matrix metalloproteinase-2 is required for 15S-hydroxyeicosatetraenoic acid-induced angiogenesis.
Singh NK, Quyen DV, Kundumani-Sridharan V, Brooks PC, Rao GN. Singh NK, et al. J Biol Chem. 2010 May 28;285(22):16830-43. doi: 10.1074/jbc.M110.106187. Epub 2010 Mar 29. J Biol Chem. 2010. PMID: 20353950 Free PMC article. - The anti-inflammatory cytokine interleukin 19 is expressed by and angiogenic for human endothelial cells.
Jain S, Gabunia K, Kelemen SE, Panetti TS, Autieri MV. Jain S, et al. Arterioscler Thromb Vasc Biol. 2011 Jan;31(1):167-75. doi: 10.1161/ATVBAHA.110.214916. Epub 2010 Oct 21. Arterioscler Thromb Vasc Biol. 2011. PMID: 20966397 Free PMC article.
References
- Sigal E, Craik CS, Highland E, et al. Molecular cloning and primary structure of human 15-lipoxygenase. Biochem Biophys Res Commun. 1988;157(2):457–464. - PubMed
- Bryant RW, Bailey JM, Schewe T, Rapoport SM. Positional specificity of a reticulocyte lipoxygenase. Conversion of arachidonic acid to 15-S-hydroperoxy-eicosatetraenoic acid. J Biol Chem. 1982;257(11):6050–6055. - PubMed
- Chang MS, Schneider C, Roberts RL, et al. Detection and subcellular localization of two 15S-lipoxygenases in human cornea. Invest Ophthalmol Vis Sci. 2005;46(3):849–856. - PubMed
- Kelavkar UP, Nixon JB, Cohen C, Dillehay D, Eling TE, Badr KF. Overexpression of 15-lipoxygenase-1 in PC-3 human prostate cancer cells increases tumorigenesis. Carcinogenesis. 2001;22(11):1765–1773. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous