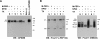Glycoprotein nonmetastatic melanoma protein b, a melanocytic cell marker, is a melanosome-specific and proteolytically released protein - PubMed (original) (raw)
Glycoprotein nonmetastatic melanoma protein b, a melanocytic cell marker, is a melanosome-specific and proteolytically released protein
Toshihiko Hoashi et al. FASEB J. 2010 May.
Abstract
Melanosomes are organelles specialized for the production of melanin pigment and are specifically produced by melanocytic cells. More than 150 pigmentation-related genes have been identified, including glycoprotein nonmetastatic melanoma protein b (GPNMB). A recent proteomics analysis revealed that GPNMB is localized in melanosomes, and GPNMB is a membrane-bound glycoprotein that shows high homology with a well-known melanosomal structural protein, Pmel17/gp100. In this study, we show that GPNMB is expressed in melanocytes of normal human skin, as well as in human melanoma cells. GPNMB is heavily glycosylated and is enriched in mature (stage III and IV) melanosomes in contrast to MART-1 and Pmel17, which are abundant in early (stage I and II) melanosomes. MART-1 and Pmel17 play critical roles in the maturation of early melanosomes; thus, we speculate that GPNMB might be important in the functions of late melanosomes, possibly their transport and/or transfer to keratinocytes. We also demonstrate that a secreted form of GPNMB is released by ectodomain shedding from the largely Golgi-modified form of GPNMB and that the PKC and Ca(2+) intracellular signaling pathways regulate that shedding. We conclude that GPNMB is a melanosomal protein that is released by proteolytic ectodomain shedding and might be a useful and specific histological marker of melanocytic cells.
Figures
Figure 1.
Domain mapping and processing of GPNMB. A) Schematic of human GPNMB and its 8 domains as defined in the text: SIG, NTD, PKD, GAP1, KRG, GAP2, TM, and CTD. Numbers represent amino acid residues based on GPNMB. Note that the RGD motif is located in the NTD. Solid circles indicate potential _N_-glycosylation sites. An isoform of GPNMB has been identified (termed GPNMB-l) where the underlined 12 aa are inserted in GAP1, probably due to alternative splicing. B) Pmel17 is subdivided into the following 10 domains as shown in A and defined in the text, including RPT. Solid circles indicate N-glycosylation sites. Dashed line indicates Furin-mediated cleavage site (CS) in GAP2, subdivided into GAP2a and GAP2b by the CS. Metalloproteinase-sensitive cleavage site (S2), γ-secretase-sensitive cleavage site (γ), multiple shedding sites in the RPT domain (broken lines) and ectodomain shedding sites (broken lines) in the GAP3 are also shown. This schematic is based on previous publications . C) Scheme of the GPNMB expression vector used; pGPNMB-tag and pGPNMB represent the human GPNMB expression vectors with or without tags, respectively. HA tag was inserted just after the SIG domain; FLAG tag was inserted just after the CTD.
Figure 2.
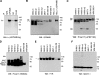
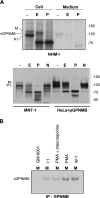
GPNMB is expressed in various types of melanocytic cells. A) HeLa cells transfected with the pGPNMB-tag were metabolically radiolabeled for 30 min. Cells were harvested immediately; extracts were then immunoprecipitated with NRS, anti-GPNMB, anti-HA, or anti-FLAG antibodies, separated by electrophoresis and visualized by autoradiography. B–F) HeLa cells transfected with the empty vector or with pGPNMB and melanocytic cells, two independent clones of NHM-l melanocytes, two independent clones of NHM-m melanocytes, highly pigmented MNT-1 cells, unpigmented SK-MEL-28 cells, and unpigmented WM266-4 cells, were harvested then solubilized. Samples were analyzed by immunoblotting using anti-GPNMB (B), αPEP13h (C), HMB45 (D), anti-TYR (E), and anti-MART-1 (F) antibodies. GPNMB is post-translationally modified in the ER (P1), then is processed to the mature form (M) in the Golgi. Pmel17 is also post-translationally modified in the ER (P1) and matures in the Golgi (to P2), then is cleaved into the Mα-Mβ complex . Mα is further processed into Mα1, Mα2, and Mα3 . Arrows indicate specific bands.
Figure 3.
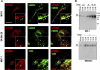
GPNMB mRNA and protein are expressed in epidermal melanocytes in vivo. A) Skin biopsies were examined by TISH using a GPNMB-specific riboprobe, as reported in Materials and Methods. B) Skin biopsies were subjected to immunohistochemistry using anti-TYR, anti-GPNMB, and anti-MART-1 antibodies. GPNMB was detected with Alexa Fluor 594 (red). TYR or MART-1 was detected with Alexa Fluor 488 (green). Nuclei were counterstained with DAPI. Broken line indicates basal membrane zone. Scale bars = 100 μm.
Figure 4.
GPNMB is preferentially detected in stage III and IV melanosomes. A) MNT-1 cells and SK-MEL-28 cells were fixed and immunostained with anti-GPNMB and HMB50 antibodies, then reacted with Alexa Fluor 594 (red) and 488 (green), respectively. MNT-1 cells were also fixed and immunostained with anti-GPNMB and TA99 antibodies, then reacted with Alexa Fluor 594 and 488, respectively. Scale bars = 20 μm. B) Subcellular fractions purified from MNT-1 cells (top) and from WM266-4 cells (bottom) were analyzed by immunoblotting using the anti-GPNMB antibody.
Figure 5.
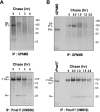
GPNMB is quickly degraded. A) MNT-1 cells were metabolically radiolabeled for 30 min, then chased for specific periods as noted. Cell lysates were immunoprecipitated with anti-GPNMB or HMB50 antibodies, separated by electrophoresis, and visualized by autoradiography. B) MNT-1 cells were metabolically radiolabeled for 15 min, then chased for specific periods as noted. Cell lysates were analyzed as described above. In vitro translation of GPNMB and Pmel17 was performed and is also shown.
Figure 6.
GPNMB is not significantly buried in the TX-insoluble fraction. MNT-1 cells were solubilized with M-PER, 1% Nonidet P-40 or 1% TX containing lysis buffer. Soluble fractions (S) and insoluble fractions (I) were analyzed by immunoblotting using anti-GPNMB (A), αPEP13h (B, left), and HMB45 (B, right) antibodies.
Figure 7.
sGPNMB is secreted into the medium. A) HeLa cells overexpressing GPNMB were metabolically radiolabeled for 30 min, then chased for 0 h (cell lysates) or 3 h (medium). Cell lysates (C) and medium (M) were immunoprecipitated with the antibodies noted and then were analyzed as described above. B) NHM-l melanocytes were metabolically radiolabeled for 30 min, then chased for specific periods as noted. Cell lysates (C) and medium (M) were immunoprecipitated with the anti-GPNMB antibody, separated by electrophoresis, and visualized by autoradiography. C) Pulse-chase experiment performed as described for B in the presence of BFA at 10 μg/ml.
Figure 8.
sGPNMB is largely Golgi modified, and its secretion is regulated ectodomain shedding. A) NHM-l melanocytes were radiolabeled and chased for 0 h (cell lysates) or for 4 h (medium), then were immunoprecipitated with the NKI/beteb antibody. Immunopurified samples were split into 3 portions; one was digested with EndoH (E), another was digested with PNGaseF (P), and the third was the untreated control. Digestion reactions were performed for 12 h at 37°C, then samples were electrophoresed and visualized by autoradiography. Bottom: MNT-1 cells and HeLa cells overexpressing GPNMB were solubilized and then digested as described above. One sample was digested with neuraminidase (N). Samples were analyzed by immunoblotting using the anti-GPNMB antibody. B) NHM-l melanocytes were radiolabeled and chased in the presence of 100 nM GM-6001, DMSO, 200 nM PMA + 100 μM staurosporine, 200 nM PMA, and 50 μM W-7. Medium was immunoprecipitated with the anti-GPNMB antibody, separated by electrophoresis, and visualized by autoradiography.
Figure 9.
Schematic of the maturation of GPNMB. GPNMB is translated as the nascent form P0. The P1 form is modified in the ER and is further modified in the Golgi to the M form. sGPNMB is produced by regulated proteolytic ectodomain shedding. Open arrowheads indicate ER-modified N-glycans; checkmarks indicate Golgi-modified _N_-glycans. Note that some checkmarked glycans might still be the high-mannose type or the hybrid-type _N_-glycan.
Similar articles
- Gpnmb is a melanosome-associated glycoprotein that contributes to melanocyte/keratinocyte adhesion in a RGD-dependent fashion.
Tomihari M, Hwang SH, Chung JS, Cruz PD Jr, Ariizumi K. Tomihari M, et al. Exp Dermatol. 2009 Jul;18(7):586-95. doi: 10.1111/j.1600-0625.2008.00830.x. Epub 2009 Mar 6. Exp Dermatol. 2009. PMID: 19320736 Free PMC article. - Silencing of GPNMB by siRNA inhibits the formation of melanosomes in melanocytes in a MITF-independent fashion.
Zhang P, Liu W, Zhu C, Yuan X, Li D, Gu W, Ma H, Xie X, Gao T. Zhang P, et al. PLoS One. 2012;7(8):e42955. doi: 10.1371/journal.pone.0042955. Epub 2012 Aug 13. PLoS One. 2012. PMID: 22912767 Free PMC article. - MART-1 is required for the function of the melanosomal matrix protein PMEL17/GP100 and the maturation of melanosomes.
Hoashi T, Watabe H, Muller J, Yamaguchi Y, Vieira WD, Hearing VJ. Hoashi T, et al. J Biol Chem. 2005 Apr 8;280(14):14006-16. doi: 10.1074/jbc.M413692200. Epub 2005 Jan 28. J Biol Chem. 2005. PMID: 15695812 - Recent advances in understanding the molecular basis of melanogenesis in melanocytes.
Ohbayashi N, Fukuda M. Ohbayashi N, et al. F1000Res. 2020 Jun 15;9:F1000 Faculty Rev-608. doi: 10.12688/f1000research.24625.1. eCollection 2020. F1000Res. 2020. PMID: 32595944 Free PMC article. Review. - Biological role of tyrosinase related protein and its biosynthesis and transport from TGN to stage I melanosome, late endosome, through gene transfection study.
Jimbow K, Gomez PF, Toyofuku K, Chang D, Miura S, Tsujiya H, Park JS. Jimbow K, et al. Pigment Cell Res. 1997 Aug;10(4):206-13. doi: 10.1111/j.1600-0749.1997.tb00486.x. Pigment Cell Res. 1997. PMID: 9263327 Review.
Cited by
- The PKD domain distinguishes the trafficking and amyloidogenic properties of the pigment cell protein PMEL and its homologue GPNMB.
Theos AC, Watt B, Harper DC, Janczura KJ, Theos SC, Herman KE, Marks MS. Theos AC, et al. Pigment Cell Melanoma Res. 2013 Jul;26(4):470-86. doi: 10.1111/pcmr.12084. Epub 2013 Apr 2. Pigment Cell Melanoma Res. 2013. PMID: 23452376 Free PMC article. - Direct observation of the effects of chemical fixation in MNT-1 cells: A SE-ADM and Raman study.
Mastrangelo R, Okada T, Ogura T, Ogura T, Baglioni P. Mastrangelo R, et al. Proc Natl Acad Sci U S A. 2023 Dec 19;120(51):e2308088120. doi: 10.1073/pnas.2308088120. Epub 2023 Dec 13. Proc Natl Acad Sci U S A. 2023. PMID: 38091295 Free PMC article. - Glycoprotein Non-Metastatic Protein B: An Emerging Biomarker for Lysosomal Dysfunction in Macrophages.
van der Lienden MJC, Gaspar P, Boot R, Aerts JMFG, van Eijk M. van der Lienden MJC, et al. Int J Mol Sci. 2018 Dec 24;20(1):66. doi: 10.3390/ijms20010066. Int J Mol Sci. 2018. PMID: 30586924 Free PMC article. Review. - Neurodegenerative phagocytes mediate synaptic stripping in Neuro-HIV.
Di Liberto G, Egervari K, Kreutzfeldt M, Schürch CM, Hewer E, Wagner I, Du Pasquier R, Merkler D. Di Liberto G, et al. Brain. 2022 Aug 27;145(8):2730-2741. doi: 10.1093/brain/awac102. Brain. 2022. PMID: 35808999 Free PMC article. - CDK1 promotes the proliferation of melanocytes in Rex rabbits.
Dai Y, Hu S, Bai S, Li J, Yang N, Zhai P, Zhao B, Chen Y, Wu X. Dai Y, et al. Genes Genomics. 2022 Oct;44(10):1191-1199. doi: 10.1007/s13258-022-01283-4. Epub 2022 Aug 11. Genes Genomics. 2022. PMID: 35951158
References
- Orlow S J. Melanosomes are specialized members of the lysosomal lineage of organelles. J Invest Dermatol. 1995;105:3–7. - PubMed
- Seiji M, Fitzpatrick T B, Simpson R T, Birbeck M S C. Chemical composition and terminology of specialized organelles (melanosomes and melanin granules) in mammalian melanocytes. Nature. 1963;197:1082–1084. - PubMed
- Kwon B S, Halaban R, Kim G S, Usack L, Pomerantz S H, Haq A K. A melanocyte-specific complementary DNA clone whose expression is inducible by melanotropin and isobutylmethyl xanthine. Mol Biol Med. 1987;4:339–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous