CRFR1 is expressed on pancreatic beta cells, promotes beta cell proliferation, and potentiates insulin secretion in a glucose-dependent manner - PubMed (original) (raw)
CRFR1 is expressed on pancreatic beta cells, promotes beta cell proliferation, and potentiates insulin secretion in a glucose-dependent manner
Mark O Huising et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Corticotropin-releasing factor (CRF), originally characterized as the principal neuroregulator of the hypothalamus-pituitary-adrenal axis, has broad central and peripheral distribution and actions. We demonstrate the presence of CRF receptor type 1 (CRFR1) on primary beta cells and show that activation of pancreatic CRFR1 promotes insulin secretion, thus contributing to the restoration of normoglycemic equilibrium. Stimulation of pancreatic CRFR1 initiates a cAMP response that promotes insulin secretion in vitro and in vivo and leads to the phosphorylation of cAMP response element binding and the induction of the expression of several immediate-early genes. Thus, the insulinotropic actions of pancreatic CRFR1 oppose the activation of CRFR1 on anterior pituitary corticotropes, leading to the release of glucocorticoids that functionally antagonize the actions of insulin. Stimulation of the MIN6 insulinoma line and primary rat islets with CRF also activates the MAPK signaling cascade leading to rapid phosphorylation of Erk1/2 in response to CRFR1-selective ligands, which induce proliferation in primary rat neonatal beta cells. Importantly, CRFR1 stimulates insulin secretion only during conditions of intermediate to high ambient glucose, and the CRFR1-dependent phosphorylation of Erk1/2 is greater with elevated glucose concentrations. This response is reminiscent of the actions of the incretins, which potentiate insulin secretion only during elevated glucose conditions. The presence of CRFR1 on beta cells adds another layer of complexity to the intricate network of paracrine and autocrine factors and their cognate receptors whose coordinated efforts can dictate islet hormone output and regulate beta cell proliferation.
Figures
Fig. 1.
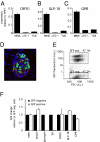
CRFR1 is expressed on insulinoma cells and on primary mouse β cells. CRFR1 expression levels are relatively abundant in the mouse insulinoma cell line MIN6 compared with the α cell line αTC-1 or the δ cell line TU6 (A). Similarly, GLP-1R (B) and GIPR (C) are expressed abundantly in the MIN6 insulinoma cells. Using dissociated primary islets from an mIP-GFP transgenic reporter mouse (D), we obtained primary β cells by FACS separation of GFP-positive and GFP-negative cells (E). The expression profile of the GFP-positive population demonstrates the retention of expression of β cell markers such as insulin, GLP-1R, and GIPR, whereas glucagon and Sst are not expressed (F). CRFR1 expression in GFP-positive cells demonstrates β cell localization of CRFR1. GFP and insulin are stained green and red, respectively, in (D); DAPI (blue) is applied as a nuclear counterstain.
Fig. 2.
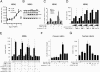
CRFR1 activation induces a cAMP response that augments GSIS in MIN6 insulinoma cells and primary rodent and human islets. CRF dose-dependently increases intracellular cAMP levels in MIN6 cells; this increase is blocked by the simultaneous administration of the CRFR1-selective antagonist antalarmin (A). Stimulation of MIN6 cells with the CRFR1-selective agonist oCRF rapidly induces robust phosphorylation of CREB (B) and leads to the stimulation of the expression of immediate-early genes including cFos, nuclear receptor subfamily 4 group A member 2 (NR4A2), regulator of G protein signaling 2 (RGS2), and dual-specificity phosphatase 1 (DUSP1) (C). These transcriptional changes are enhanced by high ambient glucose concentrations and can be blocked by the CRFR1 antagonist antalarmin. Stimulation of MIN6 cells with a relatively low dose of oCRF (1 nM) suffices to augment insulin secretion in the presence of intermediate (11 mM) or high (16.8 mM) glucose concentrations (D). The augmentation of GSIS induced by selective or preferential CRFR1 agonists (oCRF, r/hCRF, stressin1-A) is completely inhibited by general (astressin) or CRFR1-selective (antalarmin) antagonists but not by the CRFR2-selective antagonist astressin2-B (E). Stimulation with oCRF potentiates GSIS in mouse (F) and human (G) primary islets in a CRFR1-dependent manner, because coadministration of antalarmin complete blocks the effects of oCRF.
Fig. 3.
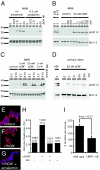
Activation of CRFR1 activates the MAPK signaling cascade and induces proliferation in rat neonatal β cells. Stimulation of CRFR1 dose-dependently increases phosphorylation of Erk1/2, an increase that is inhibited by coadministration of antalarmin (A). The induction of pErk1/2 is rapid (5 min) and persists for at least 3 h (B). oCRF and exendin-4 synergize with glucose (C). Isolated primary rat islets respond to the CRFR1-selective agonist oCRF with increased levels of pErk1/2 (D). Stimulation of rat neonatal islet cells with r/hCRF increased the nuclear incorporation of EdU into insulin-positive cells; this incorporation was blocked by antalarmin (E_–_G). Proliferation was quantified as the fraction of EdU insulin-positive cells (H). Numbers indicate the total number of insulin-positive cells; the number of insulin-positive EdU cells is given in parentheses. The total number of islets isolated from CRFR1-null pancreata was markedly lower than in control animals (I).
Fig. 4.
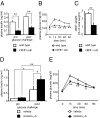
Acute stimulation of CRFR1 in vivo augments GSIS leading to enhanced glucose tolerance. Fasted plasma insulin levels in CRFR1-null males receiving a replacement dose of glucocorticoids to mitigate HPA deficiency were significantly lower than in age-matched wild-type males (A). Despite reduced fasting and glucose-induced insulin levels, glucose tolerance paradoxically was superior in CRFR1-null mice than in wild-type controls (B). CRFR1-null mice have reduced relative eWAT weight (C). Circulating insulin levels in fasted adrenalectomized wild-type animals were unaffected by prior stressin1-A administration (0.5 mg/kg i.p.) as compared with controls (D). Although glucose challenge (2 g/kg i.p.) significantly elevated circulating insulin in both groups, preadministration of stressin1-A significantly elevated GSIS (D). Stressin1-A (2.5 mg/kg, i.p.) administered 15 min before a glucose tolerance test significantly improved glucose tolerance as compared with vehicle-treated controls (E). *, P < 0.05; **, P < 0.01 vs. wild-type or vehicle-treated controls.
Similar articles
- Glucocorticoids differentially regulate the expression of CRFR1 and CRFR2α in MIN6 insulinoma cells and rodent islets.
Huising MO, Pilbrow AP, Matsumoto M, van der Meulen T, Park H, Vaughan JM, Lee S, Vale WW. Huising MO, et al. Endocrinology. 2011 Jan;152(1):138-50. doi: 10.1210/en.2010-0791. Epub 2010 Nov 24. Endocrinology. 2011. PMID: 21106875 Free PMC article. - Roles for corticotropin-releasing factor receptor type 1 in energy homeostasis in mice.
Sakamoto R, Matsubara E, Nomura M, Wang L, Kawahara Y, Yanase T, Nawata H, Takayanagi R. Sakamoto R, et al. Metabolism. 2013 Dec;62(12):1739-48. doi: 10.1016/j.metabol.2013.08.005. Epub 2013 Sep 17. Metabolism. 2013. PMID: 24054833 - Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids.
Chen A, Perrin M, Brar B, Li C, Jamieson P, Digruccio M, Lewis K, Vale W. Chen A, et al. Mol Endocrinol. 2005 Feb;19(2):441-58. doi: 10.1210/me.2004-0300. Epub 2004 Oct 28. Mol Endocrinol. 2005. PMID: 15514029 - Pancreatic Thyrotropin Releasing Hormone and Mechanism of Insulin Secretion.
Štrbák V. Štrbák V. Cell Physiol Biochem. 2018;50(1):378-384. doi: 10.1159/000494013. Epub 2018 Oct 4. Cell Physiol Biochem. 2018. PMID: 30286449 Review. - Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells.
Lee YS, Jun HS. Lee YS, et al. Metabolism. 2014 Jan;63(1):9-19. doi: 10.1016/j.metabol.2013.09.010. Epub 2013 Oct 17. Metabolism. 2014. PMID: 24140094 Review.
Cited by
- Integrating the inputs that shape pancreatic islet hormone release.
Noguchi GM, Huising MO. Noguchi GM, et al. Nat Metab. 2019 Dec;1(12):1189-1201. doi: 10.1038/s42255-019-0148-2. Epub 2019 Dec 13. Nat Metab. 2019. PMID: 32694675 Free PMC article. Review. - Loss of huntingtin-associated protein 1 impairs insulin secretion from pancreatic β-cells.
Cape A, Chen X, Wang CE, O'Neill A, Lin YF, He J, Xu XS, Yi H, Li H, Li S, Li XJ. Cape A, et al. Cell Mol Life Sci. 2012 Apr;69(8):1305-17. doi: 10.1007/s00018-011-0692-8. Epub 2011 May 5. Cell Mol Life Sci. 2012. PMID: 21544547 Free PMC article. - The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression.
Benner C, van der Meulen T, Cacéres E, Tigyi K, Donaldson CJ, Huising MO. Benner C, et al. BMC Genomics. 2014 Jul 22;15(1):620. doi: 10.1186/1471-2164-15-620. BMC Genomics. 2014. PMID: 25051960 Free PMC article. - Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets.
Ludwig B, Ziegler CG, Schally AV, Richter C, Steffen A, Jabs N, Funk RH, Brendel MD, Block NL, Ehrhart-Bornstein M, Bornstein SR. Ludwig B, et al. Proc Natl Acad Sci U S A. 2010 Jul 13;107(28):12623-8. doi: 10.1073/pnas.1005098107. Epub 2010 Jun 28. Proc Natl Acad Sci U S A. 2010. PMID: 20616039 Free PMC article. - Paracrine regulation of insulin secretion.
Huising MO. Huising MO. Diabetologia. 2020 Oct;63(10):2057-2063. doi: 10.1007/s00125-020-05213-5. Epub 2020 Sep 7. Diabetologia. 2020. PMID: 32894316 Free PMC article. Review.
References
- Kuperman Y, Chen A. Urocortins: Emerging metabolic and energy homeostasis perspectives. Trends Endocrinol Metab. 2008;19:122–129. - PubMed
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. - PubMed
- Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: Neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S50–S55. - PubMed
- Strack AM, Sebastian RJ, Schwartz MW, Dallman MF. Glucocorticoids and insulin: Reciprocal signals for energy balance. Am J Physiol. 1995;268:R142–R149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous