Antitumor activity of targeting SRC kinases in endothelial and myeloid cell compartments of the tumor microenvironment - PubMed (original) (raw)
Antitumor activity of targeting SRC kinases in endothelial and myeloid cell compartments of the tumor microenvironment
Wei Liang et al. Clin Cancer Res. 2010.
Abstract
Purpose: Several Src family kinase (SFK) inhibitors have entered clinical trials based on their direct effects against tumor cells. Here, we characterize the effects of targeting Src kinases on the tumor microenvironment and how these effects influence tumor growth.
Experimental design: Human cancer cells grown in cell culture or in mice were treated with dasatinib, a small-molecule inhibitor of SFKs. Tumor cell, endothelial cell, and myeloid cell compartments within the tumor microenvironment were analyzed. Primary human endothelial cells and freshly isolated CD11b+/CD11c- myeloid cells from mice were treated with dasatinib in cell culture. Cellular functions and signaling pathways affected by dasatinib were evaluated.
Results: Dasatinib was not cytotoxic in cell culture against the human cancer cell lines investigated here. However, dasatinib administration in human tumor-bearing mice suppressed tumor growth associated with increased tumor cell apoptosis, decreased microvessel density, and reduced intratumoral CD11b+ myeloid cells. Dasatinib directly inhibited motility and other functions of endothelial and myeloid cells, accompanied by the inhibition of phosphorylation of SFKs and downstream signaling. Tumor-infiltrating myeloid cells were identified as the major source of matrix metalloproteinase (MMP)-9 in the tumor microenvironment. Dasatinib treatment reduced MMP-9 levels in the tumor microenvironment through the simultaneous inhibition of recruitment of MMP9+ myeloid cells and MMP-9 gene expression in tumor-infiltrating myeloid cells.
Conclusions: These findings suggest that Src kinase inhibitors such as dasatinib possess a previously unrecognized anticancer mechanism of action by targeting both host-derived endothelial and myeloid cell compartments within the tumor microenvironment.
Figures
Fig. 1
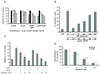
Dasatinib inhibited endothelial cell functions. A, DU145, Colo205 and HUVEC cells were treated with DMSO (control) or dasatinib for 48 h. HUVECs were serum-starved and treated with VEGF (HUVEC+VEGF) or bFGF (HUVEC+bFGF) to stimulate cell growth. Cell viability was measured by MTS assay. Average values in control (DMSO) wells were set as 100% viability. Results are expressed as mean ± SEM % of viability relative to control (n=3). SS: serum starvation. B, HUVECs were treated with DMSO or dasatinib for 48 h with or without VEGF. Cell apoptosis was measured by TUNEL assay. Results are shown as mean ± SEM % apoptotic cells (n=3). CM: complete medium. C, serum-starved HUVECs were treated with DMSO or dasatinib and allowed to migrate towards VEGF or bFGF for 6 h in a modified Boyden chamber assay. Average number of migrated cells in control (DMSO) wells was set as 100% migration. Results are expressed as mean ± SEM % of migration relative to control (n=4). D, Tube formation was quantified by counting the cord junctions of branches formed by endothelial cells. Average numbers of cord junctions in control wells (VEGF only or bFGF only) were set as 100% tube formation. Results are expressed as mean ± SEM % of tube formation relative to control (n=3).
Fig. 2
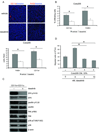
Dasatinib selectively inhibited Src downstream signaling in HUVECs. A, B and C, Serum-starved Confluent HUVECs were pretreated with DMSO or dasatinib for 1 h prior to stimulation with 50 ng/ml VEGF for another 30 min (for SFKs, FAK, p130CAS, paxillin, VE-cadherin and Stat3) or 10 min (for VEGFR2, Erk1/2, Akt and p38). Cells were lysed and cell lysate was probed with indicated antibodies. β-actin was used to demonstrate protein loading.
Fig. 3
Dasatinib inhibited human tumor growth in mouse models. Mice bearing Colo205 tumors were treated with vehicle solution or dasatinib (15 mg/kg, B.I.D., p.o.) for 21 d. A, Tumor volume was shown as mean ± SEM. (*p<0.001, n=10) B, Whole tumor lysate was isolated from 4 vehicle- or dasatinib-treated tumors and immunoblots were probed with indicated antibodies. C, Tumor sections were immunostained for p-SFKs (left and middle) and total SFKs (right). Representative images were obtained with a x40 objective. Regions surrounded by white lines were further amplified to show p-SFKs staining in endothelial cells. (Scales bars, left and right: 20 µm; middle: 5 µm.) D, Tumor sections were immunostained for CD31, TUNEL (green)/Hoechst 33342 (blue) and Ki67. Scale bars, 50 µm.
Fig. 4
Dasatinib directly inhibited tumor-associated myeloid cells. A, Upper, Colo205 tumor sections were immunostained for CD11b (red) or F4/80 (red). Representative images were obtained with a x20 objective. Scale bars, 50 µm. Lower, quantification of CD11b+ cells (*p<0.01, n=10) or F4/80+ cells per field (*p<0.01, n=10). Results are shown as mean ± SEM. B, Quantification of CD11b+ cells (*p<0.01, n=3) or F4/80+ cells (*p<0.05, n=3) by flow cytometry analyses. Results are shown as mean ± SEM % infiltrating cells. C, CD11b+/CD11c− cells were isolated from the spleens of Colo205 tumor-bearing mice treated with vehicle or dasatinib and subjected to Western blot analysis. Cell lysate was probed with indicated antibodies. D, CD11b+/CD11c− cells were isolated from the spleens of tumor-bearing mice prior to dasatinib treatment, treated with DMSO or dasatinib in vitro and allowed to migrate towards 33% tumor cell conditioned medium (CM) for 18 h in a modified Boyden Chamber assay. Results are shown as mean ± SEM × 103 migrated cells per ml medium (*p<0.001, n=8).
Fig. 5
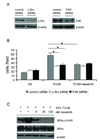
c-Src and FAK as targets for inhibition of macrophage migration. RAW264.7 cells were transiently transfected with control, c-Src or FAK siRNAs. A, lysate was prepared from RAW264.7 cells 72 h post-transfection and probed with indicated antibodies to measure the knock-down efficiency by siRNAs. B, RAW264.7 cells were harvested 72 h post-transfection, resuspended in basal medium and pretreated with DMSO or 30 nM dasatinib for 1 h before loading on the collagen I-coated inserts. Cell motility was evaluated by a modified Boyden chamber assay using 33% Colo205 tumor cell conditioned medium (TCCM) as a chemoattractant. Results were shown as mean ± SEM cells per field (x20) (*p<0.01, n=3). C, RAW264.7 cells were serum-starved for 6 h, pretreated with DMSO or dasatinib for 1 h prior to stimulation with or without 33% TCCM for another 2 h. Cell lysate was extracted and probed with indicated antibodies.
Fig. 6
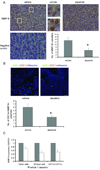
Dasatinib reduced MMP-9 levels in the tumor microenvironment. A, Upper, MMP-9 immunostaining in Colo205 xenograft tumors treated with vehicle or dasatinib. Representative images were obtained with a x40 objective. Regions surrounded by white lines were further amplified to show different MMP-9 expression pattern. (Scales bars, left and right: 20 µm; middle: 5 µm.) Lower left, negative control for MMP-9 staining. Lower right, quantification of MMP-9+ cells per field (*_p_=0.025, n=10). Results are shown as mean ± SEM. B, Upper, immunostaining of tumor sections for CD11b (red) and MMP-9 (green). Confocal fluorescence images were obtained with a x20 objective. Scale bars, 50 µm. Lower, quantification of CD11b+/MMP-9+ cells per field (*_p_=0.032, n=10). Results are shown as mean ± SEM. C, Normalized MMP-9 mRNA expression in human tumor cells, mouse stromal cells and CD11b+/CD11c− tumor-associated myeloid cells. Results are shown as mean ± SEM (n=3).
Comment in
- Targeting the tumor microenvironment with SRC kinase inhibition.
Chung AS, Ferrara N. Chung AS, et al. Clin Cancer Res. 2010 Feb 1;16(3):775-7. doi: 10.1158/1078-0432.CCR-09-3081. Epub 2010 Jan 26. Clin Cancer Res. 2010. PMID: 20103657
Similar articles
- Targeting the tumor microenvironment with SRC kinase inhibition.
Chung AS, Ferrara N. Chung AS, et al. Clin Cancer Res. 2010 Feb 1;16(3):775-7. doi: 10.1158/1078-0432.CCR-09-3081. Epub 2010 Jan 26. Clin Cancer Res. 2010. PMID: 20103657 - Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells.
Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, Mirosevich J, Lee FY, Jove R. Nam S, et al. Cancer Res. 2005 Oct 15;65(20):9185-9. doi: 10.1158/0008-5472.CAN-05-1731. Cancer Res. 2005. PMID: 16230377 - Src-kinase inhibitors sensitize human cells of myeloid origin to Toll-like-receptor-induced interleukin 12 synthesis.
Wölfl M, Schwinn S, Yoo YE, Reß ML, Braun M, Chopra M, Schreiber SC, Ayala VI, Ohlen C, Eyrich M, Beilhack A, Schlegel PG. Wölfl M, et al. Blood. 2013 Aug 15;122(7):1203-13. doi: 10.1182/blood-2013-03-488072. Epub 2013 Jul 8. Blood. 2013. PMID: 23836556 Free PMC article. - Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors.
Montero JC, Seoane S, Ocaña A, Pandiella A. Montero JC, et al. Clin Cancer Res. 2011 Sep 1;17(17):5546-52. doi: 10.1158/1078-0432.CCR-10-2616. Epub 2011 Jun 13. Clin Cancer Res. 2011. PMID: 21670084 Review. - Dasatinib: an anti-tumour agent via Src inhibition.
Gnoni A, Marech I, Silvestris N, Vacca A, Lorusso V. Gnoni A, et al. Curr Drug Targets. 2011 Apr;12(4):563-78. doi: 10.2174/138945011794751591. Curr Drug Targets. 2011. PMID: 21226671 Review.
Cited by
- Tyr724 phosphorylation of ELMO1 by Src is involved in cell spreading and migration via Rac1 activation.
Makino Y, Tsuda M, Ohba Y, Nishihara H, Sawa H, Nagashima K, Tanaka S. Makino Y, et al. Cell Commun Signal. 2015 Jul 25;13:35. doi: 10.1186/s12964-015-0113-y. Cell Commun Signal. 2015. PMID: 26205662 Free PMC article. - Improved angiostatic activity of dasatinib by modulation with hydrophobic chains.
Păunescu E, Clavel CM, Nowak-Sliwinska P, Griffioen AW, Dyson PJ. Păunescu E, et al. ACS Med Chem Lett. 2015 Jan 30;6(3):313-7. doi: 10.1021/ml500496u. eCollection 2015 Mar 12. ACS Med Chem Lett. 2015. PMID: 25815152 Free PMC article. - Carrier-free prodrug nanoparticles based on dasatinib and cisplatin for efficient antitumor in vivo.
Yang L, Xu J, Xie Z, Song F, Wang X, Tang R. Yang L, et al. Asian J Pharm Sci. 2021 Nov;16(6):762-771. doi: 10.1016/j.ajps.2021.08.001. Epub 2021 Sep 8. Asian J Pharm Sci. 2021. PMID: 35027952 Free PMC article. - Macrophage infiltration and genetic landscape of undifferentiated uterine sarcomas.
Przybyl J, Kowalewska M, Quattrone A, Dewaele B, Vanspauwen V, Varma S, Vennam S, Newman AM, Swierniak M, Bakuła-Zalewska E, Siedlecki JA, Bidzinski M, Cools J, van de Rijn M, Debiec-Rychter M. Przybyl J, et al. JCI Insight. 2017 Jun 2;2(11):e94033. doi: 10.1172/jci.insight.94033. eCollection 2017 Jun 2. JCI Insight. 2017. PMID: 28570276 Free PMC article. - Golgi phosphoprotein 3 regulates metastasis of prostate cancer via matrix metalloproteinase 9.
Li W, Qi K, Wang Z, Gu M, Chen G, Guo F, Wang Z. Li W, et al. Int J Clin Exp Pathol. 2015 Apr 1;8(4):3691-700. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26097550 Free PMC article.
References
- Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. - PubMed
- Witz IP. Yin-yang activities and vicious cycles in the tumor microenvironment. Cancer Res. 2008;68:9–13. - PubMed
- Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. - PubMed
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous