Mitochondrial respiratory chain dysfunction in dorsal root ganglia of streptozotocin-induced diabetic rats and its correction by insulin treatment - PubMed (original) (raw)
. 2010 Apr;59(4):1082-91.
doi: 10.2337/db09-1299. Epub 2010 Jan 26.
Affiliations
- PMID: 20103706
- PMCID: PMC2844817
- DOI: 10.2337/db09-1299
Mitochondrial respiratory chain dysfunction in dorsal root ganglia of streptozotocin-induced diabetic rats and its correction by insulin treatment
Subir K Roy Chowdhury et al. Diabetes. 2010 Apr.
Abstract
Objective: Impairments in mitochondrial physiology may play a role in diabetic sensory neuropathy. We tested the hypothesis that mitochondrial dysfunction in sensory neurons is due to abnormal mitochondrial respiratory function.
Research design and methods: Rates of oxygen consumption were measured in mitochondria from dorsal root ganglia (DRG) of 12- to- 22-week streptozotocin (STZ)-induced diabetic rats, diabetic rats treated with insulin, and age-matched controls. Activities and expression of components of mitochondrial complexes and reactive oxygen species (ROS) were analyzed.
Results: Rates of coupled respiration with pyruvate + malate (P + M) and with ascorbate + TMPD (Asc + TMPD) in DRG were unchanged after 12 weeks of diabetes. By 22 weeks of diabetes, respiration with P + M was significantly decreased by 31-44% and with Asc + TMPD by 29-39% compared with control. Attenuated mitochondrial respiratory activity of STZ-diabetic rats was significantly improved by insulin that did not correct other indices of diabetes. Activities of mitochondrial complexes I and IV and the Krebs cycle enzyme, citrate synthase, were decreased in mitochondria from DRG of 22-week STZ-diabetic rats compared with control. ROS levels in perikarya of DRG neurons were not altered by diabetes, but ROS generation from mitochondria treated with antimycin A was diminished compared with control. Reduced mitochondrial respiratory function was associated with downregulation of expression of mitochondrial proteins.
Conclusions: Mitochondrial dysfunction in sensory neurons from type 1 diabetic rats is associated with impaired rates of respiratory activity and occurs without a significant rise in perikaryal ROS.
Figures
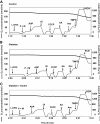
FIG. 1.
Representative measurements of oxygen consumption in isolated lumbar DRG mitochondria from control, diabetic, and insulin-treated diabetic rats. Freshly isolated lumbar DRG mitochondria were assessed in control (A), diabetic (B), and insulin-treated (C) diabetic rats using OROBOROS oxygraph 2K as described in
research design and methods
. Thick lines indicate the level of oxygen in the chamber of electrode and expressed in nanomoles per milliliter. Thin lines indicate oxygen flux per mass (picomoles O2 per second per milligram protein) in the presence of specific substrates and inhibitors of the mitochondrial respiratory chain. L-DRG, lumbar dorsal root ganglia mitochondria; P, pyruvate (10 mmol/l); M, malate (5 mmol/l); ADP, adenosine diphosphate (2 mmol/l); Ol, oligomycin (1.0 μmol/l); FCCP, carbonyl cyanide 4-trifluoromethoxyphenylhydrazone (0.5 μmol/l); AA, antimycin A (1 μg/ml); Asc, ascorbate (5 mmol/l); TMPD, N,N,N′,N′-tetramethyl-p-phenylenediamine dihydrochloride (0.5 mmol/l); KCN, potassium cyanide (0.25 mmol/l).
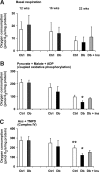
FIG. 2.
Effect of 12–22 weeks of STZ-induced diabetes on the activity of the mitochondrial respiratory chain in freshly prepared lumbar DRG tissue homogenate. Measurements of oxygen consumption were performed with energetic substrates, pyruvate and malate, as described in Fig. 1. Basal respiration (A), pyruvate + malate + ADP (coupled oxidative phosphorylation) (B), and the respiration rate with Asc + TMPD (complex IV) (C) were assessed in age-matched controls (Ctrl), STZ-diabetic rats (Db), and STZ-diabetic rats with insulin implant (Db + Ins) at 12 (n = 5), 16 (n = 7–11), and 22 (n = 5–6) weeks of diabetes. Values are means ± SD; n = as indicated. *P < 0.05 vs. Db + Ins; **P < 0.001 vs. Db (one-way ANOVA with Tukey's post hoc comparison).
FIG. 3.
Mitochondrial respiratory chain activity is impaired in freshly isolated mitochondria from lumbar DRG of 22 weeks of STZ-diabetic rats. After measurement of coupled respiration, the ATP synthase specific inhibitor, oligomycin, was added to prevent reverse pumping of protons by the ATPase, and then the uncoupled rate of mitochondrial respiration was generated by adding the uncoupling agent FCCP. Basal respiration (A), pyruvate + malate + ADP (coupled oxidative phosphorylation) (B), FCCP (uncoupled respiration) (C), and the respiration rate with Asc + TMPD (complex IV) (D) were measured in age-matched controls (Ctrl), STZ-diabetic rats (Db), and STZ-diabetic rats with insulin implant (Db + Ins) after 22 weeks of diabetes. Values are means ± SD, n = 6. *P < 0.05 vs. other groups; **P < 0.05 vs. Db + Ins (one-way ANOVA with Tukey's post hoc comparison). Treatment of the mitochondrial preparation with the uncoupler FCCP induced a four- to sixfold elevation in the rate of oxygen consumption over the basal rate confirming that the mitochondrial preparations were intact and of high quality in all groups.
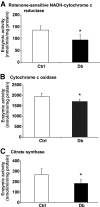
FIG. 4.
Enzymatic activities of mitochondrial respiratory chain and citrate synthase activity are decreased in isolated mitochondria from lumbar DRG of STZ-diabetic rats. Enzymatic activity of complex I was assessed as rotenone-sensitive portion of NADH-cytochrome c reductase (NCCR) (n = 5) (A), cytochrome c oxidase (n = 6–7) (B), and citrate synthase (n = 5) (C) and was measured as described in
research design and methods
. Values are means ± SD, n = as indicated. *P < 0.05 vs. control (Ctrl) (unpaired Student's t test). Db, diabetic.
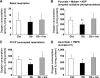
FIG. 5.
ROS were not elevated in acutely isolated DRG sensory neurons or mitochondria from STZ-diabetic rats. A–F: ROS levels were assessed using real-time fluorescence imaging of DHR 123 or CM-H2DCFDA. A and B: DHR 123 loaded neurons from acutely isolated lumbar DRG neurons of control or STZ-diabetic rats. Bar = 100 μm. C: Quantification of DHR 123 fluorescence intensities of neurons. Values are means ± SEM (n = 50–80 neurons, observed in five independently assessed control (Ctrl) and diabetic (Db) rats). D and E: CM-H2DCFDA–based ROS imaging of sensory neurons from control and STZ-diabetic rats. Bar = 50 μm. F: Quantification of DCF fluorescence emission. Values are means ± SEM (n = 200–230 neurons, observed in two independently assessed control and diabetic rats). G: ROS generation is not elevated in isolated mitochondria from lumbar DRG of diabetic rats compared with control. Mitochondrial ROS generation was measured at state 4 with substrates pyruvate and malate (P + M) in the absence or presence of antimycin A (AA). H2O2 was measured fluorometrically in 0.1 mg/ml mitochondrial suspension with Amplex Red kits (50 μmol/l Amplex Red and 0.5 units/ml horseradish peroxidase). Values are mean with n = 5. H: Fluorescence intensity at 180 min. Significance level was determined by two-way ANOVA with Bonferroni's post hoc tests. **P < 0.01 for control (P + M) vs. control (P + M + AA). (A high-quality color representation of this figure is available in the online issue.)
FIG. 6.
Expression of components of the electron transport chain are reduced in DRG from STZ-diabetic rats. Shown are representative blots (A–D) and charts in which NDUFS3 (E), COX IV (F), and ATP synthase β subunit signal (G) have been presented relative to total ERK level. Values are the means ± SEM, n = 6. **P < 0.005 vs. control (CTR), *P < 0.05 vs. Db + INS (one-way ANOVA with Tukey's post hoc test). Db, diabetic; Db + INS, diabetic with insulin implant.
Similar articles
- Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats.
Akude E, Zherebitskaya E, Chowdhury SK, Smith DR, Dobrowsky RT, Fernyhough P. Akude E, et al. Diabetes. 2011 Jan;60(1):288-97. doi: 10.2337/db10-0818. Epub 2010 Sep 28. Diabetes. 2011. PMID: 20876714 Free PMC article. - Insulin prevents aberrant mitochondrial phenotype in sensory neurons of type 1 diabetic rats.
Aghanoori MR, Smith DR, Roy Chowdhury S, Sabbir MG, Calcutt NA, Fernyhough P. Aghanoori MR, et al. Exp Neurol. 2017 Nov;297:148-157. doi: 10.1016/j.expneurol.2017.08.005. Epub 2017 Aug 10. Exp Neurol. 2017. PMID: 28803751 Free PMC article. - Sensory neurons derived from diabetic rats exhibit deficits in functional glycolysis and ATP that are ameliorated by IGF-1.
Aghanoori MR, Margulets V, Smith DR, Kirshenbaum LA, Gitler D, Fernyhough P. Aghanoori MR, et al. Mol Metab. 2021 Jul;49:101191. doi: 10.1016/j.molmet.2021.101191. Epub 2021 Feb 13. Mol Metab. 2021. PMID: 33592336 Free PMC article. - Nutrient excess and altered mitochondrial proteome and function contribute to neurodegeneration in diabetes.
Chowdhury SK, Dobrowsky RT, Fernyhough P. Chowdhury SK, et al. Mitochondrion. 2011 Nov;11(6):845-54. doi: 10.1016/j.mito.2011.06.007. Epub 2011 Jul 2. Mitochondrion. 2011. PMID: 21742060 Free PMC article. Review. - Oxidative Stress and Mitochondrial Dysfunction Associated with Peripheral Neuropathy in Type 1 Diabetes.
Eftekharpour E, Fernyhough P. Eftekharpour E, et al. Antioxid Redox Signal. 2022 Sep;37(7-9):578-596. doi: 10.1089/ars.2021.0152. Epub 2021 Dec 7. Antioxid Redox Signal. 2022. PMID: 34416846 Review.
Cited by
- Common and Divergent Mechanisms in Developmental Neuronal Remodeling and Dying Back Neurodegeneration.
Yaron A, Schuldiner O. Yaron A, et al. Curr Biol. 2016 Jul 11;26(13):R628-R639. doi: 10.1016/j.cub.2016.05.025. Curr Biol. 2016. PMID: 27404258 Free PMC article. Review. - New perspectives in diabetic neuropathy.
Eid SA, Rumora AE, Beirowski B, Bennett DL, Hur J, Savelieff MG, Feldman EL. Eid SA, et al. Neuron. 2023 Sep 6;111(17):2623-2641. doi: 10.1016/j.neuron.2023.05.003. Epub 2023 May 31. Neuron. 2023. PMID: 37263266 Free PMC article. Review. - A Role for Insulin in Diabetic Neuropathy.
Grote CW, Wright DE. Grote CW, et al. Front Neurosci. 2016 Dec 23;10:581. doi: 10.3389/fnins.2016.00581. eCollection 2016. Front Neurosci. 2016. PMID: 28066166 Free PMC article. Review. - O-GlcNAcase deficiency suppresses skeletal myogenesis and insulin sensitivity in mice through the modulation of mitochondrial homeostasis.
Wang X, Feng Z, Wang X, Yang L, Han S, Cao K, Xu J, Zhao L, Zhang Y, Liu J. Wang X, et al. Diabetologia. 2016 Jun;59(6):1287-96. doi: 10.1007/s00125-016-3919-2. Epub 2016 Mar 18. Diabetologia. 2016. PMID: 26993634 - Dorsal Root Ganglia Mitochondrial Biochemical Changes in Non-diabetic and Streptozotocin-Induced Diabetic Mice Fed with a Standard or High-Fat Diet.
Guilford BL, Ryals JM, Lezi E, Swerdlow RH, Wright DE. Guilford BL, et al. J Neurol Neurosci. 2017;8(2):180. doi: 10.21767/2171-6625.1000180. Epub 2017 Mar 27. J Neurol Neurosci. 2017. PMID: 28775932 Free PMC article.
References
- Yagihashi S: Pathogenetic mechanisms of diabetic neuropathy: lessons from animal models. J Peripher Nerv Syst 1997; 2: 113– 132 - PubMed
- Malik RA, Tesfaye S, Newrick PG, Walker D, Rajbhandari SM, Siddique I, Sharma AK, Boulton AJ, King RH, Thomas PK, Ward JD: Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia 2005; 48: 578– 585 - PubMed
- Obrosova IG: Increased sorbitol pathway activity generates oxidative stress in tissue sites for diabetic complications. Antioxid Redox Signal 2005; 7: 1543– 1552 - PubMed
- Thornalley PJ: Glycation in diabetic neuropathy: characteristics, consequences, causes, and therapeutic options. Int Rev Neurobiol 2002; 50: 37– 57 - PubMed
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M: Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000; 404: 787– 790 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous