Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions - PubMed (original) (raw)
. 2010 Mar;176(3):1169-80.
doi: 10.2353/ajpath.2010.090610. Epub 2010 Jan 28.
Dirk Deuster, Arne Knief, Michael Sperling, Michael Holtkamp, Bayram Edemir, Hermann Pavenstädt, Claudia Lanvers-Kaminsky, Antoinette am Zehnhoff-Dinnesen, Alfred H Schinkel, Hermann Koepsell, Heribert Jürgens, Eberhard Schlatter
Affiliations
- PMID: 20110413
- PMCID: PMC2832140
- DOI: 10.2353/ajpath.2010.090610
Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions
Giuliano Ciarimboli et al. Am J Pathol. 2010 Mar.
Abstract
The use of the effective antineoplastic agent cisplatin is limited by its serious side effects, such as oto- and nephrotoxicity. Ototoxicity is a problem of special importance in children, because deafness hampers their language and psychosocial development. Recently, organic cation transporters (OCTs) were identified in vitro as cellular uptake mechanisms for cisplatin. In the present study, we investigated in an in vivo model the role of OCTs in the development of cisplatin oto- and nephrotoxicity. The functional effects of cisplatin treatment on kidney (24 hours excretion of glucose, water, and protein) and hearing (auditory brainstem response) were studied in wild-type and OCT1/2 double-knockout (KO) mice. No sign of ototoxicity and only mild nephrotoxicity were observed after cisplatin treatment of knockout mice. Comedication of wild-type mice with cisplatin and the organic cation cimetidine protected from ototoxicity and partly from nephrotoxicity. For the first time we showed that OCT2 is expressed in hair cells of the cochlea. Furthermore, cisplatin-sensitive cell lines from pediatric tumors showed no expression of mRNA for OCTs, indicating the feasibility of therapeutic approaches aimed to reduce cisplatin toxicities by competing OCT2-mediated cisplatin uptake in renal proximal tubular and cochlear hair cells. These findings are very important to establish chemotherapeutical protocols aimed to maximize the antineoplastic effect of cisplatin while reducing the risk of toxicities.
Figures
Figure 1
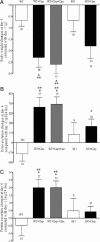
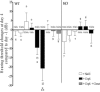
Effects of saline (white bar) or cisplatin treatment on body weight (A), 24 hours urine (B), and protein (C) excretion in wild-type (WT) and KO mice. The effects of comedication with an equimolar dose of cimetidine are also shown. Values are expressed as difference between day 4 after and day −1 before treatment. Compared with day −1, cisplatin treatment caused a significant body weight loss and a significant polyuria at day 4 in WT and KO mice and also under contemporaneous administration of cimetidine. The comparison of the effects at day 4 shows that the weight loss after cisplatin or cisplatin and cimetidine treatment of WT mice was significantly higher than in WT and KO mice treated with saline. B: WT mice treated with cisplatin or with cisplatin and cimetidine had a significantly higher urine excretion than WT mice treated with saline. C: Compared with day −1, cisplatin treatment caused a significant proteinuria at day 4 in WT and also in WT mice treated with cimetidine and cisplatin. This proteinuria was significantly higher in these two groups compared with wild-type mice treated with saline. No significant proteinuria was observed in KO mice after cisplatin injection. The number of animals tested in every group is shown for each column. *P < 0.05 paired _t_-test; **P < 0.05, analysis of varience with Newman-Keuls post-test.
Figure 2
Effects of saline (white bar) or cisplatin treatment on BUN (A) and 24 hours glucose excretion (B) in wild-type (WT) and KO mice. The effects of comedication with an equimolar dose of cimetidine are also shown. Values are expressed as difference between day 4 after and day −1 before treatment. Compared with day −1, cisplatin treatment caused a significant increase of BUN level in wild-type. In KO mice and also in wild-type mice contemporaneously given cimetidine and cisplatin, no significant BUN increase could be observed (P > 0.05, paired _t_-test). The BUN increase observed in wild-type mice treated with cisplatin was significantly higher than what observed in the other groups. Compared with day −1, cisplatin treatment caused a dramatic glucosuria at day 4 in wild-type mice. Also in KO mice and in wild-type mice comedicated with cimetidine, a significant glucosuria was detected. However, at day 4, the glucose excretion in wild-type mice was significantly higher than that observed in the other groups. Glucose excretion under treatment with cimetidine and cisplatin was significantly different from all other groups including the cisplatin treatment group. The number of animals tested in every group is shown for each column. *P < 0.05 paired _t_-test; **P < 0.05, analysis of varience with Newman-Keuls post-test; ***P < 0.05, analysis of varience with Newman-Keuls multiple comparison test.
Figure 3
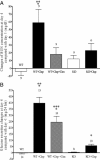
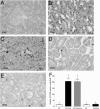
Representative photomicrographs of histology of renal corticomedullary junctions (×630) showing apoptosis induced by cisplatin. Formalin-fixed, paraffin-embedded kidneys were stained for fragmented DNA by the TUNEL method. A: Saline-treated control wild-type (WT) mouse. B: Cisplatin-treated WT mouse. C: Cisplatin + cimetidine-treated WT mouse. D: Saline-treated control KO mouse. E: Cisplatin-treated KO mouse. Apoptosis is evident after treatment of WT mice with cisplatin (B). Cotreatment with cimetidine did not suppress the induction of apoptosis (C). No apoptosis induction by cisplatin treatment was detected in KO mice (D and E). F shows the number of TUNEL-positive cells in the sections from three different kidneys for every group counted in at least 15 nonoverlapping fields/section in a blinded fashion and averaged. Significantly more apoptotic cells are evident in WT mice treated with cisplatin or cisplatin and cimetidine compared with the other groups (*P < 0.05, analysis of variance with Newman-Keuls multiple comparison test). The number of kidneys tested in every group is shown above the columns.
Figure 4
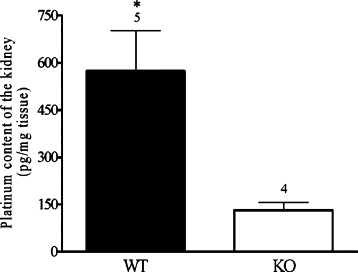
Kidney platinum content in wild-type (WT) and KO mice at day 4 after cisplatin injection. Kidneys from WT mice contain significantly more platinum than those from KO mice (*P < 0.05, unpaired _t_-test). The number of kidneys tested in every group is shown above the columns.
Figure 5
Mean changes in hearing thresholds in wild-type (WT) and KO mice treated with saline or cisplatin at the frequencies of 8, 16, and 32 kHz. The effects of comedication of WT mice with an equimolar dose of cimetidine are also shown. Four days after treatment, cisplatin caused a significant hearing loss in WT mice at 16 and also 32 kHz (*P < 0.05, paired _t_-test) compared with day −1, whereas no significant change of hearing threshold could be detected at any frequency in KO mice. Cotreatment with cimetidine suppressed the cisplatin-induced hearing loss observed in WT mice. Changes in hearing thresholds under treatment with cisplatin at the frequency of 32 kHz were significantly different from all other groups (**P < 0.05, analysis of variance with Newman-Keuls multiple comparison test). The number of animals tested in every group is shown for each column.
Figure 6
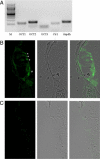
Expression of OCT and CTR1 in the cochlea. A: Representative PCR analysis of OCT and CTr1 in the mouse cochlea. M is the molecular weight marker lane. B: Immunohistochemistry of mOCT2 expression in the cochlea from a wild-type (WT) mouse. The microphotography in B (left) shows that the labeling for mOCT2 is localized both in the inner (#) and outer (*) hair cells, and also in inner spiral bundle fibers. Middle: bright-field imaging of the same cochlea; right: overlay imaging. C: Labeling of mOCT2 in KO mice. The microphotography in C (left) has been obtained using the same illumination conditions as for B (left). Bright field (middle) and overlay imaging (right) of the Corti organ. No specific labeling of inner and outer hair cells was observed.
Figure 7
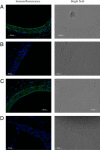
Expression of mOCT2 in the stria vascularis. Representative immunohistochemistry of mOCT2 expression in the stria vascularis of mouse cochlea from apical (A) and basal (C) turns, with the respective (B and D) negative controls in the presence of the blocking peptide. Green is the OCT2 and blue the 4′,6-diamidino-2-phenylindole (DAPI) labeling of cell nuclei. The pictures have been obtained using the same illumination conditions. On the right the respective bright field microphotographs are shown.
Figure 8
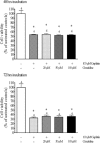
Effects of 48 (upper panel) or 72 hours (lower panel) incubation of MOLT4 cells, which do not express hOCT2, with 100 μmol/L cisplatin in the presence of 0, 20, 50, or 100 μmol/L cimetidine on the cell viability as measured with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2_H_-tetrazolium bromide proliferation assay. Cisplatin caused a significant decrease in cell viability only after 48 hours incubation. Addition of cimetidine at any concentration did not change the effect of cisplatin both after 48 and 72 hours incubation (*P < 0.05, significantly different from controls, analysis of variance with Newman-Keuls multiple comparison test).
Comment in
- Cimetidine as an organic cation transporter antagonist.
Ehrsson H, Wallin I. Ehrsson H, et al. Am J Pathol. 2010 Sep;177(3):1573-4; author reply 1574. doi: 10.2353/ajpath.2010.100484. Epub 2010 Jul 22. Am J Pathol. 2010. PMID: 20651231 Free PMC article.
Similar articles
- Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity.
Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Filipski KK, et al. Clin Pharmacol Ther. 2009 Oct;86(4):396-402. doi: 10.1038/clpt.2009.139. Epub 2009 Jul 22. Clin Pharmacol Ther. 2009. PMID: 19625999 Free PMC article. - Selective Inhibition on Organic Cation Transporters by Carvedilol Protects Mice from Cisplatin-Induced Nephrotoxicity.
Guo D, Yang H, Li Q, Bae HJ, Obianom O, Zeng S, Su T, Polli JE, Shu Y. Guo D, et al. Pharm Res. 2018 Sep 6;35(11):204. doi: 10.1007/s11095-018-2486-2. Pharm Res. 2018. PMID: 30191328 Free PMC article. - Ondansetron can enhance cisplatin-induced nephrotoxicity via inhibition of multiple toxin and extrusion proteins (MATEs).
Li Q, Guo D, Dong Z, Zhang W, Zhang L, Huang SM, Polli JE, Shu Y. Li Q, et al. Toxicol Appl Pharmacol. 2013 Nov 15;273(1):100-9. doi: 10.1016/j.taap.2013.08.024. Epub 2013 Aug 31. Toxicol Appl Pharmacol. 2013. PMID: 24001450 Free PMC article. - Membrane transporters as mediators of cisplatin side-effects.
Ciarimboli G. Ciarimboli G. Anticancer Res. 2014 Jan;34(1):547-50. Anticancer Res. 2014. PMID: 24403515 Review. - Saving ears and kidneys from cisplatin.
Wensing KU, Ciarimboli G. Wensing KU, et al. Anticancer Res. 2013 Oct;33(10):4183-8. Anticancer Res. 2013. PMID: 24122981 Review.
Cited by
- Comparison of Anticancer Drug Toxicities: Paradigm Shift in Adverse Effect Profile.
Basak D, Arrighi S, Darwiche Y, Deb S. Basak D, et al. Life (Basel). 2021 Dec 29;12(1):48. doi: 10.3390/life12010048. Life (Basel). 2021. PMID: 35054441 Free PMC article. Review. - Role of Mouse Organic Cation Transporter 2 for Nephro- and Peripheral Neurotoxicity Induced by Chemotherapeutic Treatment with Cisplatin.
Hucke A, Schröter R, Ceresa C, Chiorazzi A, Canta A, Semperboni S, Marmiroli P, Cavaletti G, Gess B, Ciarimboli G. Hucke A, et al. Int J Mol Sci. 2023 Jul 14;24(14):11486. doi: 10.3390/ijms241411486. Int J Mol Sci. 2023. PMID: 37511245 Free PMC article. - Pharmacogenomics of cisplatin-induced ototoxicity.
Mukherjea D, Rybak LP. Mukherjea D, et al. Pharmacogenomics. 2011 Jul;12(7):1039-50. doi: 10.2217/pgs.11.48. Pharmacogenomics. 2011. PMID: 21787192 Free PMC article. Review. - Emerging Roles of the Human Solute Carrier 22 Family.
Yee SW, Giacomini KM. Yee SW, et al. Drug Metab Dispos. 2021 Dec 17;50(9):1193-210. doi: 10.1124/dmd.121.000702. Online ahead of print. Drug Metab Dispos. 2021. PMID: 34921098 Free PMC article. - Mechanisms of Cisplatin nephrotoxicity.
Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Miller RP, et al. Toxins (Basel). 2010 Nov;2(11):2490-518. doi: 10.3390/toxins2112490. Epub 2010 Oct 26. Toxins (Basel). 2010. PMID: 22069563 Free PMC article. Review.
References
- Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. - PubMed
- Skinner R. Best practice in assessing ototoxicity in children with cancer. Eur J Cancer. 2004;40:2352–2354. - PubMed
- Ramirez-Camacho R, Garcia-Berrocal JR, Trinidad A, Verdaguer JM, Nevado J. Blebs in inner and outer hair cells: a pathophysiological hypothesis. J Laryngol Otol. 2008;122:1151–1155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials