CDK inhibitor p21 is prosurvival in adriamycin-induced podocyte injury, in vitro and in vivo - PubMed (original) (raw)
CDK inhibitor p21 is prosurvival in adriamycin-induced podocyte injury, in vitro and in vivo
Caroline B Marshall et al. Am J Physiol Renal Physiol. 2010 May.
Abstract
In response to injury, the highly specialized and terminally differentiated glomerular visceral epithelial cell, or podocyte, may undergo several cell fates, including dedifferentiation and proliferation, persistent cell cycle arrest, hypertrophy, apoptosis, or necrosis. Common to these potential outcomes of injury is their ultimate regulation at the level of the cell cycle. There is now a large body of literature confirming the importance of cell cycle regulatory proteins in the cellular response to injury. Although CDK inhibitor p21 levels increase in podocytes following injury, the role of p21 is unclear in focal segmental glomerulosclerosis (FSGS), in part because its function depends heavily on the cytotoxic stimulus and the cellular context. Adriamycin (ADR) is a podocyte toxin used to induce experimental FSGS. The purpose of this study was to define the role of p21 in ADR-induced podocyte injury. BALB/c mice, a strain carrying the recessive ADR susceptibility gene, were backcrossed against c57B6 p21-/- mice to yield a 12th generation BALB/c p21-/- strain. Experimental FSGS was induced by injection of ADR 12 mg/kg × 2 doses (n = 8/group), with mice killed at 1, 2, 8, and 11 wk. Diseased p21-/- mice demonstrated worse albuminuria, more widespread glomerulosclerosis, and higher blood urea nitrogen compared with diseased p21+/+ mice. In diseased p21-/- mice vs. p21+/+ mice, apoptosis [measured by TdT-mediated dUTP nick end labeling (TUNEL) assay] was increased, and podocyte number (measured by WT-1 immunostaining) was decreased. To validate these findings in vitro, we utilized differentiated mouse podocytes, p21-/- and p21+/+, exposed to 0.125 μg/ml ADR. Apoptosis, measured by Hoechst 33342 staining and TUNEL assay, was greater in cultured p21-/- podocytes compared with p21+/+ podocytes. Reconstitution of p21 via retroviral transfection rescued the p21-/- podocytes from apoptosis. We conclude that p21 is prosurvival in the podocyte's response to ADR-induced injury. Ongoing studies are defining the mechanisms of this protective effect as it relates to DNA damage and apoptosis.
Figures
Fig. 1.
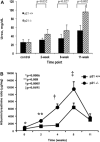
Trend of weights in p21+/+ and p21−/− mice following treatment with Adriamycin (ADR). A: weight loss trend. At baseline, before disease induction, there is no statistically significant difference between the mean body weights of p21+/+ and p21−/− mice. Following the injections, by weeks 1 and 6, p21−/− mice lost more weight to a significant degree. Other time points do not demonstrate significant differences. B and C: weights of individual mice at each time point during the 11-wk study. B: p21+/+ animals. C: p21−/− animals.
Fig. 2.
Plasma blood urea nitrogen and urinary albumin-to-creatinine ratio in p21+/+ and p21−/− mice following treatment with ADR. A: plasma blood urinary nitrogen. By 8 wk, diseased p21−/− mice had a statistically significantly greater plasma blood urinary nitrogen level than diseased p21+/+ mice. The difference remained statistically significant at 11 wk following disease induction (P < 0.05). B: urinary albumin-to-creatinine ratio. At baseline, 2 wk, 4 wk, and 8 wk, diseased p21−/− mice demonstrated statistically significantly greater levels of albumin-to-creatinine ratio than diseased p21+/+ mice (P < 0.05). By 11 wk, the difference was no longer statistically significant.
Fig. 3.
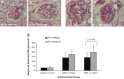
Histopathologic changes by periodic acid-Schiff (PAS) staining in p21+/+ and p21−/− mice following treatment with ADR. A and B: histopathology (original magnification ×40). Representative micrographs of PAS staining of tissues from p21+/+ mice are shown, time points 8 and 11 wk following disease induction. C and D: histopathology (original magnification ×40). Representative micrographs of PAS staining of tissues from p21−/− mice are shown, time points 8 and 11 wk following disease induction. Tissue from diseased p21−/− mice exhibited greater disease severity than tissue from diseased p21+/+ mice. E: sclerosis index. Tissue from diseased p21−/− mice exhibited statistically significantly greater disease severity compared with tissue from diseased p21+/+ mice, as semiquantitatively assessed by sclerosis index, at 11 wk following disease induction (P < 0.05).
Fig. 4.
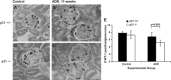
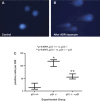
WT-1 immunostaining in p21+/+ and p21−/− mice following treatment with ADR. By immunohistochemistry (original magnification ×40), the number of WT-1-positive cells was decreased significantly in tissues from p21−/− mice 11 wk following disease induction compared with time-matched diseased p21+/+ mice (arrows indicate examples of positive cells; P < 0.05). A: representative immunostaining for WT-1, a surrogate marker for podocyte number, in tissue from p21+/+ mice that received vehicle only. B: representative immunostaining for WT-1 in tissue from p21+/+ mice 11 wk following disease induction. C: representative immunostaining for WT-1 in tissue from p21−/− mice that received vehicle only. D: representative immunostaining for WT-1 in tissue from p21−/− mice 11 wk following disease induction. E: quantification of WT-1-positive cells per glomerulus shown in graphic form (all glomeruli counted per section × 8 animals in each group).
Fig. 5.
Apoptosis detection in p21+/+ and p21−/− mice following treatment with ADR. TdT-mediated dUTP nick end labeling (TUNEL) staining of tissue sections. Representative images of tissue sections from mice 1 wk following disease induction in p21+/+ group (A) vs. p21−/− group (B). C and D: quantification of TUNEL-positive cells per 200 glomeruli counted. Following disease induction, there was a statistically significantly greater number of TUNEL-positive cells in tissue from diseased p21−/− mice compared with tissue from diseased p21+/+ mice at 1 wk (P < 0.05; C) and at 2 wk (P < 0.05; D).
Fig. 6.
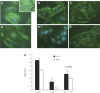
Apoptosis detection in p21+/+ and p21−/− podocytes in culture following treatment with ADR. A_–_D: representative images of podocytes stained with Hoechst 33342 following treatment with ADR (A and C: p21+/+ podocytes; B and D: p21−/− podocytes; A and B: original magnification ×10; C and D: original magnification ×20). E: quantification of Hoechst 33342-positive podocytes in culture per 300 cells counted. By 18 h, both p21+/+ and p21−/− podocytes in culture exhibited statistically significant levels of apoptosis compared with their respective baseline levels (P < 0.05). By 48 h, there was a statistically significant difference in the number of apoptotic cells by Hoechst staining between p21+/+ and p21−/− podocytes, with greater number of Hoechst-positive cells in the p21−/− podocytes (P < 0.05). Following reconstitution of p21 in p21−/− podocytes via retroviral transfection, there was a statistically significant decrease in Hoechst-positive cells 48 h after ADR treatment in the p21−/− podocytes with reconstituted p21 vs. the p21−/− podocytes (P < 0.05). F: quantification of TUNEL-positive podocytes in culture per 1,000 cells counted. At 48 h following ADR treatment, there was a statistically significantly greater number of TUNEL-positive cells in the p21−/− podocytes compared with the p21+/+ podocytes (P < 0.05). Following reconstitution of p21 via retroviral transfection, the number of TUNEL-positive cells was decreased significantly in the p21−/− podocytes with reconstituted p21 vs. the p21−/− podocytes (P < 0.05).
Fig. 7.
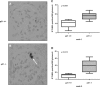
Increased DNA damage in p21−/− podocytes in culture following treatment with ADR. Damaged DNA was measured by the CometAssay in cultured podocytes following exposure to ADR at 48 h (original magnification ×20). A: representative micrograph of fluorescent DNA stain of control cells, showing undamaged and supercoiled DNA remaining within the nuclear cell membrane. B: representative micrograph of fluorescent DNA stain of ADR-treated podocytes, showing denatured DNA fragments migrating out from the cell in a long “comet tail.” C: quantification of podocytes with long comet tails. Following exposure to ADR, there was a statistically significant increase in the number of p21−/− podocytes with long comet tails compared with that seen in p21+/+ podocytes. Reconstitution of p21 significantly reduced the level of DNA damage (P < 0.05).
Fig. 8.
Diminished F-actin staining in p21−/− podocytes in culture at baseline and following treatment with ADR. The actin cytoskeleton was labeled with FITC-conjugated phalloidin to assess for actin cytoskeletal changes in podocytes following treatment with ADR. A: at baseline, before ADR exposure, the p21+/+ podocytes exhibit a central group of thick and well-defined actin stress fibers (inset shows close-up image; magnification ×20). B and C: before exposure, the p21−/− podocytes and p21−/− podocytes transfected with p21 exhibit decreased actin stress fibers compared with p21+/+ podocytes. D: following 48 h of treatment with ADR, there is decreased intensity of the actin stress fiber staining in p21+/+ podocytes. E: decrease in actin stress fibers is more marked in the p21−/− podocytes following ADR-induced injury. F: in p21−/− podocytes reconstituted with p21, there is less attenuation of actin stress fibers with injury. G: quantification of actin score, shown in graphic form. Within each group, upon treatment with ADR, the podocytes significantly lose actin stress fibers (P < 0.05). Compared with p21+/+ podocytes, p21−/− podocytes lose significantly more actin stress fibers. This loss is significantly reduced in the p21−/− podocytes with reconstituted p21 (P < 0.05).
Similar articles
- Rosuvastatin protects against podocyte apoptosis in vitro.
Cormack-Aboud FC, Brinkkoetter PT, Pippin JW, Shankland SJ, Durvasula RV. Cormack-Aboud FC, et al. Nephrol Dial Transplant. 2009 Feb;24(2):404-12. doi: 10.1093/ndt/gfn528. Epub 2008 Sep 27. Nephrol Dial Transplant. 2009. PMID: 18820279 Free PMC article. - Genetic podocyte lineage reveals progressive podocytopenia with parietal cell hyperplasia in a murine model of cellular/collapsing focal segmental glomerulosclerosis.
Suzuki T, Matsusaka T, Nakayama M, Asano T, Watanabe T, Ichikawa I, Nagata M. Suzuki T, et al. Am J Pathol. 2009 May;174(5):1675-82. doi: 10.2353/ajpath.2009.080789. Epub 2009 Apr 9. Am J Pathol. 2009. PMID: 19359523 Free PMC article. - Angiopoietin-like-3 knockout protects against glomerulosclerosis in murine adriamycin-induced nephropathy by attenuating podocyte loss.
Dai R, Liu H, Han X, Liu J, Zhai Y, Rao J, Shen Q, Xu H. Dai R, et al. BMC Nephrol. 2019 May 24;20(1):185. doi: 10.1186/s12882-019-1383-1. BMC Nephrol. 2019. PMID: 31126248 Free PMC article. - The podocyte's response to injury: role in proteinuria and glomerulosclerosis.
Shankland SJ. Shankland SJ. Kidney Int. 2006 Jun;69(12):2131-47. doi: 10.1038/sj.ki.5000410. Epub 2006 May 10. Kidney Int. 2006. PMID: 16688120 Review. - Cell cycle regulatory proteins in podocyte health and disease.
Marshall CB, Shankland SJ. Marshall CB, et al. Nephron Exp Nephrol. 2007;106(2):e51-9. doi: 10.1159/000101793. Epub 2007 Jun 6. Nephron Exp Nephrol. 2007. PMID: 17570940 Review.
Cited by
- New Mutation of Coenzyme Q10 Monooxygenase 6 Causing Podocyte Injury in a Focal Segmental Glomerulosclerosis Patient.
Song CC, Hong Q, Geng XD, Wang X, Wang SQ, Cui SY, Guo MD, Li O, Cai GY, Chen XM, Wu D. Song CC, et al. Chin Med J (Engl). 2018 Nov 20;131(22):2666-2675. doi: 10.4103/0366-6999.245158. Chin Med J (Engl). 2018. PMID: 30425193 Free PMC article. - Albumin-induced apoptosis of glomerular parietal epithelial cells is modulated by extracellular signal-regulated kinase 1/2.
Chang AM, Ohse T, Krofft RD, Wu JS, Eddy AA, Pippin JW, Shankland SJ. Chang AM, et al. Nephrol Dial Transplant. 2012 Apr;27(4):1330-43. doi: 10.1093/ndt/gfr483. Epub 2011 Sep 5. Nephrol Dial Transplant. 2012. PMID: 21896500 Free PMC article. - Ret is critical for podocyte survival following glomerular injury in vivo.
Hou G, Wu V, Singh G, Holzman LB, Tsui CC. Hou G, et al. Am J Physiol Renal Physiol. 2015 Apr 1;308(7):F774-83. doi: 10.1152/ajprenal.00483.2014. Epub 2015 Jan 13. Am J Physiol Renal Physiol. 2015. PMID: 25587123 Free PMC article. - CaMKII may regulate renal tubular epithelial cell apoptosis through YAP/NFAT2 in acute kidney injury mice.
Huang Z, Peng Y, Ke G, Xiao Y, Chen Y. Huang Z, et al. Ren Fail. 2023 Dec;45(1):2172961. doi: 10.1080/0886022X.2023.2172961. Ren Fail. 2023. PMID: 36718671 Free PMC article. - New insights into the pathology of podocyte loss: mitotic catastrophe.
Liapis H, Romagnani P, Anders HJ. Liapis H, et al. Am J Pathol. 2013 Nov;183(5):1364-1374. doi: 10.1016/j.ajpath.2013.06.033. Epub 2013 Sep 3. Am J Pathol. 2013. PMID: 24007883 Free PMC article. Review.
References
- Al-Douahji M, Brugarolas J, Brown PA, Stehman-Breen CO, Alpers CE, Shankland SJ. The cyclin kinase inhibitor p21WAF1/CIP1 is required for glomerular hypertrophy in experimental diabetic nephropathy. Kidney Int 56: 1691–1699, 1999 - PubMed
- Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377: 552–557, 1995 - PubMed
- Cao W, Chi WH, Wang J, Tang JJ, Lu YJ. TNF-alpha promotes Doxorubicin-induced cell apoptosis and anti-cancer effect through downregulation of p21 in p53-deficient tumor cells. Biochem Biophys Res Commun 330: 1034–1040, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials